Predictors of iron versus erythropoietin responsiveness in anemic hemodialysis patients
- PMID: 35833334
- PMCID: PMC9796788
- DOI: 10.1111/hdi.13030
Predictors of iron versus erythropoietin responsiveness in anemic hemodialysis patients
Abstract
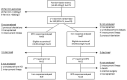
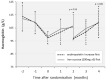
Anemia protocols for hemodialysis patients usually titrate erythropoietin (ESA) according to hemoglobin and iron according to a threshold of ferritin, with variable response seen. A universally optimum threshold for ferritin may be incorrect, and another view is that ESA and iron are alternative anemia treatments, which should be selected based on the likely response to each. Hemodialysis patients developing moderate anemia were randomised to treatment with either an increase in ESA or a course of intravenous iron. Over 2423 patient-months in 197 patients, there were 133 anemia episodes with randomized treatment. Treatment failure was seen in 20/66 patients treated with ESA and 20/67 patients treated with iron (30.3 vs. 29.9%, p = 1.0). Successful ESA treatment was associated with lower C-reactive protein (13.5 vs. 28.6 mg/L, p = 0.038) and lower previous ESA dose (6621 vs. 9273 μg/week, p = 0.097). Successful iron treatment was associated with lower reticulocyte hemoglobin (33.8 vs. 35.5 pg, p = 0.047), lower hepcidin (91.4 vs. 131.0 μg/ml, p = 0.021), and higher C-reactive protein (29.5 vs. 12.6 mg/L, p = 0.085). A four-variable iron preference score was developed to indicate the more favorable treatment, which in a retrospective analysis reduced treatment failure to 17%. Increased ESA and iron are equally effective, though treatment failure occurs in almost 30%. Baseline variables including hepcidin can predict treatment response, and a four-variable score shows promise in allowing directed treatment with improved response rates.
Keywords: anemia; hemodialysis; hepcidin; iron.
© 2022 The Authors. Hemodialysis International published by Wiley Periodicals LLC on behalf of International Society for Hemodialysis.
Conflict of interest statement
This study was funded by a Clinical Research Fellowship awarded by Imperial College Healthcare NHS Trust, and a project grant from Imperial Health Charity. FT is supported by the Diamond Fund from Imperial College Healthcare Charity, and the Ken and Mary Minton Chair of Renal Medicine. Infrastructure support for this research was provided by NIHR Imperial Biomedical Research Centre (BRC). DA has received a speaker's honorarium from Fibrogen. FT has received research project grants from AstraZeneca Limited, Baxter Biosciences, Boehringer Ingelheim, and MedImmune. He also has consultancy agreements with Baxter Biosciences, Novartis, Rigel Pharmaceuticals and UCB.
Figures


References
-
- Van Stone JC. Who should receive recombinant human erythropoietin? Semin Nephrol. 1989;9(Suppl 2):S3–7. - PubMed
-
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–90. - PubMed
-
- Oliveira C, Boquinhas H, Gaspar A, Adragão T, Boquinhas JM, Júnior EC, et al. Assessment of iron requirements during treatment of anemia with recombinant human erythropoietin in patients with chronic renal insufficiency under hemodialysis. Acta Med Port. 1992;5(7):351–7. - PubMed
-
- Susantitaphong P, Alqahtani F, Jaber BL. Efficacy and safety of intravenous iron therapy for functional iron deficiency anemia in hemodialysis patients: a meta‐analysis. Am J Nephrol. 2014;39(2):130–41. - PubMed
-
- Zhang Y, Thamer M, Stefanik K, Kaufman J, Cotter DJ. Epoetin requirements predict mortality in hemodialysis patients. Am J Kidney Dis. 2004;44(5):866–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

