Niemann-Pick type C disease as proof-of-concept for intelligent biomarker panel selection in neurometabolic disorders
- PMID: 35833379
- PMCID: PMC9796541
- DOI: 10.1111/dmcn.15334
Niemann-Pick type C disease as proof-of-concept for intelligent biomarker panel selection in neurometabolic disorders
Abstract
Aim: Using Niemann-Pick type C disease (NPC) as a paradigm, we aimed to improve biomarker discovery in patients with neurometabolic disorders.
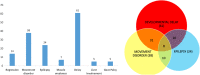
Method: Using a multiplexed liquid chromatography tandem mass spectrometry dried bloodspot assay, we developed a selective intelligent biomarker panel to monitor known biomarkers N-palmitoyl-O-phosphocholineserine and 3β,5α,6β-trihydroxy-cholanoyl-glycine as well as compounds predicted to be affected in NPC pathology. We applied this panel to a clinically relevant paediatric patient cohort (n = 75; 35 males, 40 females; mean age 7 years 6 months, range 4 days-19 years 8 months) presenting with neurodevelopmental and/or neurodegenerative pathology, similar to that observed in NPC.
Results: The panel had a far superior performance compared with individual biomarkers. Namely, NPC-related established biomarkers used individually had 91% to 97% specificity but the combined panel had 100% specificity. Moreover, multivariate analysis revealed long-chain isoforms of glucosylceramide were elevated and very specific for patients with NPC.
Interpretation: Despite advancements in next-generation sequencing and precision medicine, neurological non-enzymatic disorders remain difficult to diagnose and lack robust biomarkers or routine functional testing for genetic variants of unknown significance. Biomarker panels may have better diagnostic accuracy than individual biomarkers in neurometabolic disorders, hence they can facilitate more prompt disease identification and implementation of emerging targeted, disease-specific therapies.
What this paper adds: Intelligent biomarker panel design can help expedite diagnosis in neurometabolic disorders. In Niemann-Pick type C disease, such a panel performed better than individual biomarkers. Biomarker panels are easy to implement and widely applicable to neurometabolic conditions.
© 2022 The Authors. Developmental Medicine & Child Neurology published by John Wiley & Sons Ltd on behalf of Mac Keith Press.
Figures


Comment in
-
Intelligent biomarker panel development for neurometabolic disease.Dev Med Child Neurol. 2022 Dec;64(12):1441-1442. doi: 10.1111/dmcn.15380. Epub 2022 Aug 17. Dev Med Child Neurol. 2022. PMID: 35978465 No abstract available.
References
-
- Schulz A, Ajayi T, Specchio N, de Los Reyes E, Gissen P, Ballon D, et al. Study of Intraventricular Cerliponase Alfa for CLN2 Disease. The New England journal of medicine. 2018;378(20):1898–907. - PubMed
-
- Mengel E, Patterson MC, Da Riol RM, Del Toro M, Deodato F, Gautschi M, et al. Efficacy and safety of arimoclomol in Niemann‐Pick disease type C: Results from a double‐blind, randomised, placebo‐controlled, multinational phase 2/3 trial of a novel treatment. Journal of inherited metabolic disease. 2021;44(6):1463–80. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

