(4+3) Annulation of Donor-Acceptor Cyclopropanes and Azadienes: Highly Stereoselective Synthesis of Azepanones
- PMID: 35833420
- PMCID: PMC9545371
- DOI: 10.1002/anie.202209006
(4+3) Annulation of Donor-Acceptor Cyclopropanes and Azadienes: Highly Stereoselective Synthesis of Azepanones
Abstract
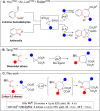
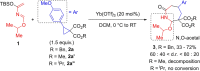
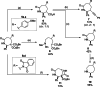
Azepanes are important seven-membered heterocycles, which are present in numerous natural and synthetic compounds. However, the development of convergent synthetic methods to access them remains challenging. Herein, we report the Lewis acid catalyzed (4+3) annulative addition of aryl and amino donor-acceptor cyclopropanes with 2-aza-1,3-dienes. Densely substituted azepane derivatives were obtained in good to excellent yields and with high diastereoselectivity. The reaction occurred under mild conditions with ytterbium triflate as the catalyst. The use of copper triflate with a trisoxazoline (Tox) ligand led to an enantioselective transformation. The obtained cycloadducts were convenient substrates for a series of further modifications, showing the synthetic utility of these compounds.
Keywords: Azadienes; Azepanones; Cycloadditions; Cyclopropanes; Tox-Ligands.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Diastereoselective [3 + 2] Cycloaddition of Quinoxalin-2(1H)-ones with Donor-Acceptor Cyclopropanes: Efficient Synthesis of Tetrahydro pyrrolo[1,2-a]quinoxalin-4(5H)-ones.J Org Chem. 2021 Dec 3;86(23):16558-16572. doi: 10.1021/acs.joc.1c01872. Epub 2021 Nov 15. J Org Chem. 2021. PMID: 34780178
-
(3+3)-Annulation of Donor-Acceptor Cyclopropanes with Diaziridines.Angew Chem Int Ed Engl. 2018 Aug 6;57(32):10338-10342. doi: 10.1002/anie.201805258. Epub 2018 Jul 17. Angew Chem Int Ed Engl. 2018. PMID: 29936708
-
Divergent Reactivity of Thioalkynes in Lewis Acid Catalyzed Annulations with Donor-Acceptor Cyclopropanes.Chemistry. 2016 Aug 16;22(34):11997-2001. doi: 10.1002/chem.201602755. Epub 2016 Jul 19. Chemistry. 2016. PMID: 27431096
-
Donor-Acceptor Cyclopropanes in the Synthesis of Carbocycles.Chem Rec. 2019 Nov;19(11):2189-2208. doi: 10.1002/tcr.201800166. Epub 2019 Feb 1. Chem Rec. 2019. PMID: 30707497 Review.
-
Rhodium-Catalyzed (5 + 2) and (5 + 1) Cycloadditions Using 1,4-Enynes as Five-Carbon Building Blocks.Acc Chem Res. 2020 Jan 21;53(1):231-243. doi: 10.1021/acs.accounts.9b00477. Epub 2019 Dec 10. Acc Chem Res. 2020. PMID: 31820914 Free PMC article. Review.
Cited by
-
Synthesis of β-enamino malonates through caesium carbonate-promoted reaction of nitro-substituted donor-acceptor cyclopropanes.RSC Adv. 2024 Oct 22;14(45):33587-33591. doi: 10.1039/d4ra05619a. eCollection 2024 Oct 17. RSC Adv. 2024. PMID: 39439834 Free PMC article.
-
Donor-Acceptor Cyclopropanes: Activation Enabled by a Single, Vinylogous Acceptor.Angew Chem Int Ed Engl. 2023 Jan 2;62(1):e202214390. doi: 10.1002/anie.202214390. Epub 2022 Nov 30. Angew Chem Int Ed Engl. 2023. PMID: 36322458 Free PMC article.
-
Asymmetric ring-opening reactions of donor-acceptor cyclopropanes with 1,3-cyclodiones.RSC Adv. 2023 Mar 7;13(11):7432-7435. doi: 10.1039/d2ra08257h. eCollection 2023 Mar 1. RSC Adv. 2023. PMID: 36895764 Free PMC article.
-
Synthesis of 1-aryl-2,3-diaroyl cyclopropanes from 1,3,5-triaryl-1,5-diketones and their transformation into E,E-1,4-diaryl-1,3-butadienes.RSC Adv. 2024 Jul 12;14(31):22076-22085. doi: 10.1039/d4ra02525c. eCollection 2024 Jul 12. RSC Adv. 2024. PMID: 39005250 Free PMC article.
References
-
- Devon T. K., Scott A. I., Handbook of Naturally Occurring Compounds, Vol. 2, Academic Press, New York, 1972.
-
- None
-
- Smalley R. K., Comprehensive Heterocyclic Chemistry, Vol. 7, Pergamon, Oxford, 1984, pp. 491–546;
-
- Kaur M., Garg S., Malhi S. D., Sohal S. H., Curr. Org. Chem. 2021, 25, 449.
-
- None
Grants and funding
LinkOut - more resources
Full Text Sources

