Hepatic patatin-like phospholipase domain-containing 3 levels are increased in I148M risk allele carriers and correlate with NAFLD in humans
- PMID: 35833455
- PMCID: PMC9512469
- DOI: 10.1002/hep4.2032
Hepatic patatin-like phospholipase domain-containing 3 levels are increased in I148M risk allele carriers and correlate with NAFLD in humans
Abstract
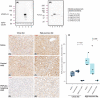
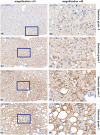
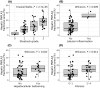
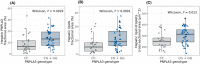
In nonalcoholic fatty liver disease (NAFLD) the patatin-like phospholipase domain-containing 3 (PNPLA3) rs738409 variant is a contributor. In mice, the Pnpla3 148M variant accumulates on lipid droplets and probably leads to sequestration of a lipase cofactor leading to impaired mobilization of triglycerides. To advance our understanding of the localization and abundance of PNPLA3 protein in humans, we used liver biopsies from patients with NAFLD to investigate the link to NAFLD and the PNPLA3 148M genotype. We experimentally qualified an antibody against human PNPLA3. Hepatic PNPLA3 protein fractional area and localization were determined by immunohistochemistry in biopsies from a well-characterized NAFLD cohort of 67 patients. Potential differences in hepatic PNPLA3 protein levels among patients related to degree of steatosis, lobular inflammation, ballooning, and fibrosis, and PNPLA3 I148M gene variants were assessed. Immunohistochemistry staining in biopsies from patients with NAFLD showed that hepatic PNPLA3 protein was predominantly localized to the membranes of small and large lipid droplets in hepatocytes. PNPLA3 protein levels correlated strongly with steatosis grade (p = 0.000027) and were also significantly higher in patients with lobular inflammation (p = 0.009), ballooning (p = 0.022), and significant fibrosis (stage 2-4, p = 0.014). In addition, PNPLA3 levels were higher in PNPLA3 rs738409 148M (CG, GG) risk allele carriers compared to 148I (CC) nonrisk allele carriers (p = 0.0029). Conclusion: PNPLA3 protein levels were associated with increased hepatic lipid content and disease severity in patients with NAFLD and were higher in PNPLA3 rs738409 (148M) risk allele carriers. Our hypothesis that increased hepatic levels of PNPLA3 may be part of the pathophysiological mechanism of NAFLD is supported.
© 2022 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Dr. Ericson, Dr. Carlsson, Dr. Andréasson, Dr. Dix, Dr. Linden, Dr. Knochel, Dr. Schumi, Dr. Bergenholm, Dr. Antonsson, Dr. Liljebad, Dr. Fjellstrom, and Dr. Hansson are employed by and own stock in AstraZeneca. Dr. Ekstedt advises AMRA Medical AB, has received lecture fee from Olink, and received unrestricted grants from Intercept, AstraZeneca, and Gilead. Dr. Lee is employed by Verve and owns stock in Verve and Ionis Pharmaceuticals. Dr. Kechagias has received lecture fees from Gilead, AbbVie, and MSD. Dr. Nasr has nothing to report.
Figures

Similar articles
-
Experimental Models to Investigate PNPLA3 in Liver Steatosis.Liver Int. 2025 May;45(5):e70091. doi: 10.1111/liv.70091. Liver Int. 2025. PMID: 40231787 Free PMC article. Review.
-
Pnpla3 silencing with antisense oligonucleotides ameliorates nonalcoholic steatohepatitis and fibrosis in Pnpla3 I148M knock-in mice.Mol Metab. 2019 Apr;22:49-61. doi: 10.1016/j.molmet.2019.01.013. Epub 2019 Feb 5. Mol Metab. 2019. PMID: 30772256 Free PMC article.
-
The rs2294918 E434K variant modulates patatin-like phospholipase domain-containing 3 expression and liver damage.Hepatology. 2016 Mar;63(3):787-98. doi: 10.1002/hep.28370. Epub 2016 Jan 14. Hepatology. 2016. PMID: 26605757
-
Human hepatocyte PNPLA3-148M exacerbates rapid non-alcoholic fatty liver disease development in chimeric mice.Cell Rep. 2022 Sep 13;40(11):111321. doi: 10.1016/j.celrep.2022.111321. Cell Rep. 2022. PMID: 36103835 Free PMC article.
-
PNPLA3 I148M and response to treatment for hepatic steatosis: A systematic review.Liver Int. 2023 May;43(5):975-988. doi: 10.1111/liv.15533. Epub 2023 Feb 16. Liver Int. 2023. PMID: 36719059
Cited by
-
Experimental Models to Investigate PNPLA3 in Liver Steatosis.Liver Int. 2025 May;45(5):e70091. doi: 10.1111/liv.70091. Liver Int. 2025. PMID: 40231787 Free PMC article. Review.
-
PNPLA3 I148M variant links to adverse metabolic traits in MASLD during fasting and feeding.JHEP Rep. 2025 May 10;7(8):101450. doi: 10.1016/j.jhepr.2025.101450. eCollection 2025 Aug. JHEP Rep. 2025. PMID: 40677694 Free PMC article.
-
Gut microbes, diet, and genetics as drivers of metabolic liver disease: a narrative review outlining implications for precision medicine.J Nutr Biochem. 2024 Nov;133:109704. doi: 10.1016/j.jnutbio.2024.109704. Epub 2024 Jul 17. J Nutr Biochem. 2024. PMID: 39029595 Review.
-
Identification of NUV-244 as a PNPLA3 I148M degrading small molecule.iScience. 2025 Apr 8;28(5):112384. doi: 10.1016/j.isci.2025.112384. eCollection 2025 May 16. iScience. 2025. PMID: 40322074 Free PMC article.
-
Isoschaftoside in Fig Leaf Tea Alleviates Nonalcoholic Fatty Liver Disease in Mice via the Regulation of Macrophage Polarity.Nutrients. 2025 Feb 21;17(5):757. doi: 10.3390/nu17050757. Nutrients. 2025. PMID: 40077628 Free PMC article.
References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy‐proven NAFLD. J Hepatol. 2017;67:1265–73. - PubMed
-
- Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology. 2020;158:1611–25.e12. - PubMed
-
- Bedossa P, FLIP Pathology Consortium . Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–75. - PubMed

