Postnatal eye size in mice is controlled by SREBP2-mediated transcriptional repression of Lrp2 and Bmp2
- PMID: 35833708
- PMCID: PMC9382895
- DOI: 10.1242/dev.200633
Postnatal eye size in mice is controlled by SREBP2-mediated transcriptional repression of Lrp2 and Bmp2
Abstract
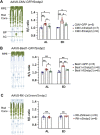
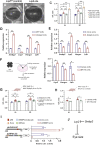
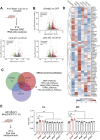
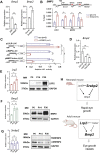
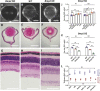
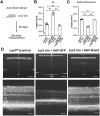
Eye size is a key parameter of visual function, but the precise mechanisms of eye size control remain poorly understood. Here, we discovered that the lipogenic transcription factor sterol regulatory element-binding protein 2 (SREBP2) has an unanticipated function in the retinal pigment epithelium (RPE) to promote eye size in postnatal mice. SREBP2 transcriptionally represses low density lipoprotein receptor-related protein 2 (Lrp2), which has been shown to restrict eye overgrowth. Bone morphogenetic protein 2 (BMP2) is the downstream effector of Srebp2 and Lrp2, and Bmp2 is suppressed by SREBP2 transcriptionally but activated by Lrp2. During postnatal development, SREBP2 protein expression in the RPE decreases whereas that of Lrp2 and Bmp2 increases as the eye growth rate reduces. Bmp2 is the key determinant of eye size such that its level in mouse RPE inversely correlates with eye size. Notably, RPE-specific Bmp2 overexpression by adeno-associated virus effectively prevents the phenotypes caused by Lrp2 knock out. Together, our study shows that rapid postnatal eye size increase is governed by an RPE-derived signaling pathway, which consists of both positive and negative regulators of eye growth.
Keywords: Bmp2; LRP2; Eye; Organ size; Retinal pigment epithelium; SREBP2.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
Foxg1-Cre Mediated Lrp2 Inactivation in the Developing Mouse Neural Retina, Ciliary and Retinal Pigment Epithelia Models Congenital High Myopia.PLoS One. 2015 Jun 24;10(6):e0129518. doi: 10.1371/journal.pone.0129518. eCollection 2015. PLoS One. 2015. PMID: 26107939 Free PMC article.
-
Selective Ablation of Megalin in the Retinal Pigment Epithelium Results in Megaophthalmos, Macromelanosome Formation and Severe Retina Degeneration.Invest Ophthalmol Vis Sci. 2019 Jan 2;60(1):322-330. doi: 10.1167/iovs.18-25667. Invest Ophthalmol Vis Sci. 2019. PMID: 30665232 Free PMC article.
-
Impaired vitreous composition and retinal pigment epithelium function in the FoxG1::LRP2 myopic mice.Biochim Biophys Acta Mol Basis Dis. 2017 Jun;1863(6):1242-1254. doi: 10.1016/j.bbadis.2017.03.022. Epub 2017 Mar 31. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28366874
-
Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium.Invest Ophthalmol Vis Sci. 2012 Sep 12;53(10):6072-80. doi: 10.1167/iovs.12-9917. Invest Ophthalmol Vis Sci. 2012. PMID: 22879416 Free PMC article.
-
Generation of a homozygous LRP2 knockout human embryonic stem cell line (FDCHDPe010-A-56) by CRISPR/Cas9 system.Stem Cell Res. 2021 May;53:102342. doi: 10.1016/j.scr.2021.102342. Epub 2021 Apr 8. Stem Cell Res. 2021. PMID: 33878707 Free PMC article.
Cited by
-
Emerging role of liver-bone axis in osteoporosis.J Orthop Translat. 2024 Sep 4;48:217-231. doi: 10.1016/j.jot.2024.07.008. eCollection 2024 Sep. J Orthop Translat. 2024. PMID: 39290849 Free PMC article. Review.
-
LRP2 is a potential molecular target for nonsyndromic pathological myopia.JCI Insight. 2025 Jun 24;10(15):e192929. doi: 10.1172/jci.insight.192929. eCollection 2025 Aug 8. JCI Insight. 2025. PMID: 40553567 Free PMC article.
-
Insights into Myopia from Mouse Models.Annu Rev Vis Sci. 2024 Sep;10(1):213-238. doi: 10.1146/annurev-vision-102122-102059. Epub 2024 Sep 2. Annu Rev Vis Sci. 2024. PMID: 38635876 Free PMC article. Review.
-
Bone morphogenetic proteins (BMPs) at the forefront of ocular diseases and therapeutics.Eye Vis (Lond). 2025 Jul 23;12(1):29. doi: 10.1186/s40662-025-00445-1. Eye Vis (Lond). 2025. PMID: 40696418 Free PMC article. Review.
-
Lipid Metabolism Regulators Are the Possible Determinant for Characteristics of Myopic Human Scleral Stroma Fibroblasts (HSSFs).Int J Mol Sci. 2023 Dec 29;25(1):501. doi: 10.3390/ijms25010501. Int J Mol Sci. 2023. PMID: 38203671 Free PMC article.
References
-
- Amemiya-Kudo, M., Shimano, H., Hasty, A. H., Yahagi, N., Yoshikawa, T., Matsuzaka, T., Okazaki, H., Tamura, Y., Iizuka, Y., Ohashi, K.et al. (2002). Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J. Lipid Res. 43, 1220-1235. 10.1194/jlr.M100417-JLR200 - DOI - PubMed
-
- Bainbridge, J. W. B., Smith, A. J., Barker, S. S., Robbie, S., Henderson, R., Balaggan, K., Viswanathan, A., Holder, G. E., Stockman, A., Tyler, N.et al. (2008). Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358, 2231-2239. 10.1056/NEJMoa0802268 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

