Cas12a-Capture: A Novel, Low-Cost, and Scalable Method for Targeted Sequencing
- PMID: 35833801
- PMCID: PMC9419982
- DOI: 10.1089/crispr.2021.0140
Cas12a-Capture: A Novel, Low-Cost, and Scalable Method for Targeted Sequencing
Abstract
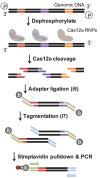
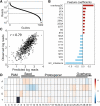
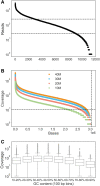
Targeted sequencing remains a valuable technique for clinical and research applications. However, many existing technologies suffer from pervasive guanine-cytosine (GC) sequence content bias, high input DNA requirements, and high cost for custom panels. We have developed Cas12a-Capture, a low-cost and highly scalable method for targeted sequencing. The method utilizes preprogrammed guide RNAs to direct CRISPR-Cas12a cleavage of double-stranded DNA in vitro and then takes advantage of the resulting four to five nucleotide overhangs for selective ligation with a custom sequencing adapter. Addition of a second sequencing adapter and enrichment for ligation products generates a targeted sequence library. We first performed a pilot experiment with 7176 guides targeting 3.5 Mb of DNA. Using these data, we modeled the sequence determinants of Cas12a-Capture efficiency, then designed an optimized set of 11,438 guides targeting 3.0 Mb. The optimized guide set achieves an average 64-fold enrichment of targeted regions with minimal GC bias. Cas12a-Capture variant calls had strong concordance with Illumina Platinum Genome calls, especially for single nucleotide variants, which could be improved by applying basic variant quality heuristics. We believe Cas12a-Capture has a wide variety of potential clinical and research applications and is amendable for selective enrichment for any double-stranded DNA template or genome.
Conflict of interest statement
Oregon Health & Science University, T.L.M., C.A.T., A.A., and B.J.O. have submitted a patent application for the Cas12a-Capture method, PCT/US2020/049966.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

