Molecular crowding facilitates bundling of IMPDH polymers and cytoophidium formation
- PMID: 35833994
- PMCID: PMC11072341
- DOI: 10.1007/s00018-022-04448-2
Molecular crowding facilitates bundling of IMPDH polymers and cytoophidium formation
Abstract
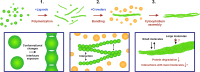
The cytoophidium is a unique type of membraneless compartment comprising of filamentous protein polymers. Inosine monophosphate dehydrogenase (IMPDH) catalyzes the rate-limiting step of de novo GTP biosynthesis and plays critical roles in active cell metabolism. However, the molecular regulation of cytoophidium formation is poorly understood. Here we show that human IMPDH2 polymers bundle up to form cytoophidium-like aggregates in vitro when macromolecular crowders are present. The self-association of IMPDH polymers is suggested to rely on electrostatic interactions. In cells, the increase of molecular crowding with hyperosmotic medium induces cytoophidia, while the decrease of that by the inhibition of RNA synthesis perturbs cytoophidium assembly. In addition to IMPDH, CTPS and PRPS cytoophidium could be also induced by hyperosmolality, suggesting a universal phenomenon of cytoophidium-forming proteins. Finally, our results indicate that the cytoophidium can prolong the half-life of IMPDH, which is proposed to be one of conserved functions of this subcellular compartment.
Keywords: Cellular compartmentalization; Cytoophidium; IMPDH; Membraneless organelle; Molecular crowding.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures







References
-
- Liu JL. The cytoophidium and its kind: filamentation and compartmentation of metabolic enzymes. Annu Rev Cell Dev Biol. 2016;32:349–372. - PubMed
-
- Liu JL. Intracellular compartmentation of CTP synthase in Drosophila. J Genet Genom. 2010;37(5):281–296. - PubMed
-
- Zhang B, Tastan OY, Zhou X, Guo CJ, Liu X, Thind A, Hu HH, Zhao S, Liu JL. The proline synthesis enzyme P5CS forms cytoophidia in Drosophila. J Genet Genom. 2020;47(3):131–143. - PubMed
MeSH terms
Substances
Grants and funding
- MC_U137788471/MRC_/Medical Research Council/United Kingdom
- MC_UU_12021/3/MRC_/Medical Research Council/United Kingdom
- 2021YFA0804701-4/Ministry of Science and Technology of the People's Republic of China
- 31771490/National Natural Science Foundation of China
- 20JC1410500/Shanghai Science and Technology Commission
LinkOut - more resources
Full Text Sources

