Impact of Partial Meal Replacement on Glycemic Levels and Body Weight in Indian Patients with Type 2 Diabetes (PRIDE): A Randomized Controlled Study
- PMID: 35834107
- PMCID: PMC9281377
- DOI: 10.1007/s13300-022-01294-0
Impact of Partial Meal Replacement on Glycemic Levels and Body Weight in Indian Patients with Type 2 Diabetes (PRIDE): A Randomized Controlled Study
Abstract
Introduction: Partial meal replacement (PMR) offers potential glycemic and weight control benefits in type 2 diabetes mellitus (T2DM) patients. We evaluated the clinical impact of PMR (diabetes-specific nutritional supplement [DSNS]) in overweight/obese Indian patients with T2DM.
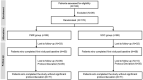
Methods: PRIDE, a 12-week, phase IV, open-label, multicenter study randomized (1:1) newly diagnosed T2DM patients (≤ 1 year) to either DSNS plus standard of care (SOC; diabetes treatment with dietary counseling) group (PMR) or SOC alone group (SOC). The primary endpoint was mean change in glycated hemoglobin (HbA1c) from baseline to week 12. Secondary endpoints were changes in glucose profiles, body weight, waist circumference, lipid profile, and factors impacting quality-of-life (QoL) at week 6 and 12 from baseline. Safety was assessed throughout the study.
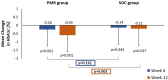
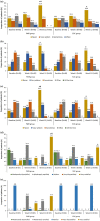
Results: Of the 176 patients enrolled, 171 (n = 85 in PMR group; n = 86 in SOC group) were included in the modified intent-to-treat population. The mean reduction in HbA1c at week 12 from baseline in PMR group was significant compared to the SOC group (- 0.59 vs. - 0.21%, p = 0.002). At week 12, the PMR group showed significant reduction in mean body weight (- 2.19 vs. - 0.22 kg; p = 0.001) and waist circumference (- 2.34 vs. - 0.48 cm; p = 0.001) compared to SOC group. Mean fasting plasma glucose and post-prandial glucose significantly reduced from baseline at week 6 and 12 in each group (p < 0.05). No significant change was observed in lipid profile. QoL parameters (treatment adherence, general well-being, and energy fulfilment) in the PMR were significantly better than SOC group (p < 0.05). Patients were satisfied with the taste of DSNS. No serious adverse events were reported.
Conclusions: DSNS is an encouraging option for PMR strategy, as it significantly improved HbA1c, body weight, waist circumference, and overall well-being among overweight/obese Indian T2DM patients.
Trial identification no: CTRI/2019/10/021595.
Keywords: Body weight; Diabetes-specific nutritional supplement; Glycemic control; India; Partial meal replacement; Quality of life; Type 2 diabetes mellitus.
© 2022. The Author(s).
Figures


Similar articles
-
A Pilot Study Evaluating the Effects of Diabetes Specific Nutrition Supplement and Lifestyle Intervention on Glycemic Control in Overweight and Obese Asian Indian Adults with Type 2 Diabetes Mellitus.J Assoc Physicians India. 2019 Dec;67(12):25-30. J Assoc Physicians India. 2019. PMID: 31801326 Clinical Trial.
-
How best to use partial meal replacement in managing overweight or obese patients with poorly controlled type 2 diabetes.Obesity (Silver Spring). 2013 Feb;21(2):251-3. doi: 10.1002/oby.20057. Obesity (Silver Spring). 2013. PMID: 23404963 Clinical Trial.
-
Partial Meal Replacement for Weight Loss after Stroke: Results of a Pilot Clinical Trial.Cerebrovasc Dis. 2024;53(1):54-61. doi: 10.1159/000530996. Epub 2023 May 15. Cerebrovasc Dis. 2024. PMID: 37231793 Clinical Trial.
-
The inclusion of a partial meal replacement with or without inulin to a calorie restricted diet contributes to reach recommended intakes of micronutrients and decrease plasma triglycerides: a randomized clinical trial in obese Mexican women.Nutr J. 2012 Jun 18;11:44. doi: 10.1186/1475-2891-11-44. Nutr J. 2012. PMID: 22703579 Free PMC article. Clinical Trial.
-
Use of a diabetes-specific nutritional shake to replace a daily breakfast and afternoon snack improves glycemic responses assessed by continuous glucose monitoring in people with type 2 diabetes: a randomized clinical pilot study.BMJ Open Diabetes Res Care. 2020 Jul;8(1):e001258. doi: 10.1136/bmjdrc-2020-001258. BMJ Open Diabetes Res Care. 2020. PMID: 32718934 Free PMC article. Clinical Trial.
Cited by
-
Culturally Tailored Dietary Interventions for Improving Glycaemic Control and Preventing Complications in South Asians with Type 2 Diabetes: Success and Future Implications.Healthcare (Basel). 2023 Apr 13;11(8):1123. doi: 10.3390/healthcare11081123. Healthcare (Basel). 2023. PMID: 37107956 Free PMC article. Review.
-
Diabetes-specific formula with standard of care improves glycemic control, body composition, and cardiometabolic risk factors in overweight and obese adults with type 2 diabetes: results from a randomized controlled trial.Front Nutr. 2024 Jul 15;11:1400580. doi: 10.3389/fnut.2024.1400580. eCollection 2024. Front Nutr. 2024. PMID: 39077157 Free PMC article.
-
Meal replacements on obesity and leptin: a systematic review and meta-analysis.Rev Endocr Metab Disord. 2025 Feb;26(1):55-80. doi: 10.1007/s11154-024-09918-5. Epub 2024 Oct 21. Rev Endocr Metab Disord. 2025. PMID: 39433654
References
-
- Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072 - PubMed
Associated data
LinkOut - more resources
Full Text Sources

