The Economic Benefit of Remission for Patients with Rheumatoid Arthritis
- PMID: 35834162
- PMCID: PMC9510082
- DOI: 10.1007/s40744-022-00473-6
The Economic Benefit of Remission for Patients with Rheumatoid Arthritis
Abstract
Introduction: In patients with rheumatoid arthritis (RA), attaining remission or low disease activity (LDA), as recommended by the treat-to-target approach, has shown to yield improvement in symptoms and quality of life. However, limited evidence from real-world settings is available to support the premise that better disease control is associated with lower healthcare costs. This study fills in evidence gaps regarding the cost of care by RA disease activity (DA) states and by therapy.
Methods: This retrospective cohort study linked medical and prescription claims from Optum Clinformatics Data Mart to electronic health record data from Illumination Health over 1/1/2010-3/31/2020. Mean annual costs for payers and patients were examined, stratifying on DA state and baseline use of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), biologics, and targeted synthetic (ts)DMARDs. Subgroup analysis examining within-person change in costs pre- and post-initiation of new therapy was also performed. Descriptive statistics, means, and boot-strapped confidence intervals were analyzed by DA state and by RA therapy. Furthermore, multivariate negative binomial regression analysis adjusting for key baseline characteristics was conducted.
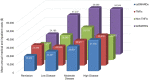
Results: Of 2339 eligible patients, 19% were in remission, 40% in LDA, 29% in moderate DA (MDA), and 12% in high DA (HDA) at baseline. Mean annual costs during follow-up were substantially less for patients in remission ($40,072) versus those in MDA ($56,536) and HDA ($59,217). For patients in remission, csDMARD use was associated with the lowest mean annual cost ($25,575), tsDMARD was highest ($75,512), and tumor necrosis factor inhibitor (TNFi) ($69,846) and non-TNFi ($57,507) were intermediate. Among new TNFi (n = 137) and non-TNFi initiators (n = 107), 31% and 26% attained LDA/remission, respectively, and the time to achieve remission/LDA was numerically shorter in TNFi vs. non-TNFi initiators. For those on biologics, mean annual within-person medical and inpatient costs were lower after achieving LDA/remission, although pharmacy costs were higher.
Conclusions: Cost of care increased with increasing DA state, with patients in remission having the lowest costs. Optimizing DA has the potential for substantial savings in healthcare costs, although may be partially offset by the high cost of targeted RA therapies.
Keywords: Cost of care; Disease activity; Tumor necrosis factor inhibitors; bDMARDs; csDMARDs; tsDMARDs.
© 2022. The Author(s).
Figures

Similar articles
-
Real-world patient characteristics and use of disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: a cross-national study.Clin Rheumatol. 2023 Apr;42(4):1047-1059. doi: 10.1007/s10067-022-06478-4. Epub 2022 Dec 19. Clin Rheumatol. 2023. PMID: 36534353 Free PMC article.
-
Incidence of dementia in patients with rheumatoid arthritis and association with disease modifying anti-rheumatic drugs - Analysis of a national claims database.Semin Arthritis Rheum. 2022 Dec;57:152083. doi: 10.1016/j.semarthrit.2022.152083. Epub 2022 Aug 17. Semin Arthritis Rheum. 2022. PMID: 36155968
-
Factors influencing prescribing the first add-on disease-modifying antirheumatic drugs in patients initiating methotrexate for rheumatoid arthritis.Explor Res Clin Soc Pharm. 2023 Jun 15;11:100296. doi: 10.1016/j.rcsop.2023.100296. eCollection 2023 Sep. Explor Res Clin Soc Pharm. 2023. PMID: 37521021 Free PMC article.
-
Singapore Chapter of Rheumatologists updated consensus statement on the eligibility for government subsidization of biologic and targeted-synthetic therapy for the treatment of rheumatoid arthritis.Int J Rheum Dis. 2020 Feb;23(2):140-152. doi: 10.1111/1756-185X.13762. Epub 2019 Dec 19. Int J Rheum Dis. 2020. PMID: 31859424 Review.
-
Treating to target in established rheumatoid arthritis: Challenges and opportunities in an era of novel targeted therapies and biosimilars.Best Pract Res Clin Rheumatol. 2015 Aug-Dec;29(4-5):543-9. doi: 10.1016/j.berh.2015.10.001. Best Pract Res Clin Rheumatol. 2015. PMID: 26697765 Review.
Cited by
-
Household catastrophic health expenditures for rheumatoid arthritis: a single centre study from South India.Sci Rep. 2023 Sep 16;13(1):15385. doi: 10.1038/s41598-023-42623-y. Sci Rep. 2023. PMID: 37717053 Free PMC article.
-
A Real-World Comparison of Clinical Effectiveness in Patients with Rheumatoid Arthritis Treated with Upadacitinib, Tumor Necrosis Factor Inhibitors, and Other Advanced Therapies After Switching from an Initial Tumor Necrosis Factor Inhibitor.Adv Ther. 2024 Sep;41(9):3706-3721. doi: 10.1007/s12325-024-02948-0. Epub 2024 Aug 7. Adv Ther. 2024. PMID: 39110310 Free PMC article.
-
An Access-Focused Patient-Centric Value Assessment Framework for Medication Formulary Decision-Making in Immune-Mediated Inflammatory Diseases.Adv Ther. 2025 Feb;42(2):568-578. doi: 10.1007/s12325-024-03076-5. Epub 2024 Dec 20. Adv Ther. 2025. PMID: 39704878 Free PMC article.
-
One-Year Medication Adherence and Persistence in Rheumatoid Arthritis in Clinical Practice: A Retrospective Analysis of Upadacitinib, Adalimumab, Baricitinib, and Tofacitinib.Adv Ther. 2023 Oct;40(10):4493-4503. doi: 10.1007/s12325-023-02619-6. Epub 2023 Aug 5. Adv Ther. 2023. PMID: 37542646 Free PMC article.
-
Generating Real-World Evidence From the Excellence Network in Rheumatology.Pharmacoepidemiol Drug Saf. 2024 Dec;33(12):e70067. doi: 10.1002/pds.70067. Pharmacoepidemiol Drug Saf. 2024. PMID: 39662998
References
LinkOut - more resources
Full Text Sources

