Efficacy and Safety of Ciprofloxacin Plus Fluocinolone Acetonide Among Patients With Acute Otitis Externa: A Randomized Clinical Trial
- PMID: 35834251
- PMCID: PMC10881221
- DOI: 10.1001/jamanetworkopen.2022.21699
Efficacy and Safety of Ciprofloxacin Plus Fluocinolone Acetonide Among Patients With Acute Otitis Externa: A Randomized Clinical Trial
Abstract
Importance: Ciprofloxacin, 0.3%, plus fluocinolone acetonide, 0.025%, otic solution seems to be efficacious and safe in treating acute otitis externa (AOE) compared with ciprofloxacin, 0.3%, or fluocinolone acetonide, 0.025%, otic solution alone.
Objective: To evaluate the superiority of ciprofloxacin, 0.3%, plus fluocinolone acetonide, 0.025%, otic solution compared with ciprofloxacin, 0.3%, or fluocinolone acetonide, 0.025%, otic solution alone in treating AOE.
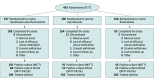
Design, setting, and participants: A phase 3 randomized, double-blind, active-controlled clinical trial was conducted between August 1, 2017, and September 14, 2018, at 36 centers in the US. The study population comprised 493 patients aged 6 months or older with AOE of less than 21 days' duration with otorrhea, moderate or severe otalgia, and edema, as well as a Brighton grading of II or III (tympanic membrane obscure but without systemic illness). Statistical analysis was performed from November 14, 2018, to February 14, 2019.
Interventions: Participants were randomly assigned to receive ciprofloxacin plus fluocinolone, ciprofloxacin, or fluocinolone twice daily for 7 days and were evaluated on day 1 (visit 1; baseline), days 3 to 4 (visit 2; conducted via telephone), days 8 to 10 (visit 3; end of treatment), and days 15 to 17 (visit 4; test of cure).
Main outcomes and measures: The primary outcome was therapeutic cure (clinical and microbiological) at the end of the treatment period. The principal secondary end point was the time to end of ear pain. Efficacy analyses were conducted in the microbiological intent-to-treat population, clinical intent-to-treat population, and microbiological intent-to-treat population with Pseudomonas aeruginosa and Staphylococcus aureus.
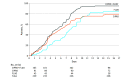
Results: A total of 493 patients (254 female patients [51.5%]; mean [SD] age, 38.2 [23.1] years) were randomized (197 to receive ciprofloxacin plus fluocinolone, 196 to receive ciprofloxacin, and 100 to receive fluocinolone). Therapeutic cure in the modified intent-to-treat population with ciprofloxacin plus fluocinolone (63 of 103 [61.2%]) was statistically comparable to that of ciprofloxacin (49 of 91 [53.8%]; difference in response rate, 7.3%; 95% CI, -6.6% to 21.2%; P = .30) and fluocinolone (20 of 45 [44.4%]; difference in response rate, 16.7%; 95% CI, -0.6% to 34.0%; P = .06) at visit 3 and significantly superior to ciprofloxacin at visit 4 (90 of 103 [87.4%] vs 69 of 91 [75.8%]; difference in response rate, 11.6%; 95% CI, 0.7%-22.4%; P = .04). A statistically faster resolution of otalgia was achieved among patients treated with ciprofloxacin plus fluocinolone (median, 5.0 days [range, 4.2-6.3 days]) vs ciprofloxacin (median, 5.9 days [range, 4.3-7.3 days]; 95% CI, 4.3-7.3 days; P = .002) or fluocinolone (median, 7.7 days [range, 6.7-9.0 days]; 95% CI, 6.7-9.0 days; P < .001). Ciprofloxacin plus fluocinolone demonstrated statistical superiority in sustained microbiological response vs ciprofloxacin (94 of 103 [91.3%] vs 74 of 91 [81.3%]; difference in response rate, 9.9%; 95% CI, 0.3%-19.6%; P = .04) and fluocinolone (34 of 45 [75.6%]; difference in response rate, 15.7%; 95% CI, 2.0%-29.4%; P = .01) and in the microbiological outcome vs fluocinolone by visit 3 (99 of 103 [96.1%] vs 37 of 45 [82.2%]; difference in response rate, 13.9%; 95% CI, 2.1%-25.7%; P = .01) and ciprofloxacin by visit 4 (97 of 103 [94.2%] vs 77 of 91 [84.6%]; difference in response rate, 9.6%; 95% CI, 0.9%-18.2%; P = .02). Fifteen adverse events related to study medications were registered, all of which were mild or moderate.
Conclusions and relevance: Ciprofloxacin, 0.3%, plus fluocinolone acetonide, 0.025%, otic solution was efficacious and safe in treating AOE but did not demonstrate superiority vs ciprofloxacin, 0.3%, or fluocinolone acetonide, 0.025%, otic solutions alone in the main study end point of therapeutic cure.
Trial registration: ClinicalTrials.gov Identifier: NCT03196973.
Conflict of interest statement
Figures
References
-
- Sander R. Otitis externa: a practical guide to treatment and prevention. Am Fam Physician. 2001;63(5):927-936, 941-942. - PubMed
-
- Guthrie RM, Bailey BJ, Witsell DL, et al. . Diagnosis and treatment of acute otitis externa: an interdisciplinary update: introduction. Ann Otol Rhinol Laryngol. 1999;108(2 suppl):2-18. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

