Risk of Mild Cognitive Impairment or Probable Dementia in New Users of Angiotensin II Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors: A Secondary Analysis of Data From the Systolic Blood Pressure Intervention Trial (SPRINT)
- PMID: 35834254
- PMCID: PMC9284332
- DOI: 10.1001/jamanetworkopen.2022.20680
Risk of Mild Cognitive Impairment or Probable Dementia in New Users of Angiotensin II Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors: A Secondary Analysis of Data From the Systolic Blood Pressure Intervention Trial (SPRINT)
Abstract
Importance: The cardiovascular and renal outcomes of angiotensin-II receptor blocker (ARB) and angiotensin-converting enzyme inhibitor (ACEI) treatment are well-known; however, few studies have evaluated initiation of these agents and cognitive impairment.
Objective: To emulate a target trial to evaluate the cognitive outcomes of initiating an ARB- vs ACEI-based antihypertensive regimen in individuals at risk for mild cognitive impairment (MCI) and probable dementia (PD).
Design, setting, and participants: Active comparator, new-user observational cohort study design using data from the Systolic Blood Pressure Intervention Trial (SPRINT), conducted November 2010 through July 2018. Marginal cause-specific hazard ratios (HRs) and treatment-specific cumulative incidence functions were estimated with inverse probability (IP) weighting to account for confounding. Participants were using neither an ARB nor ACEI at baseline. Data analysis was conducted from April 7, 2021, to April 26, 2022.
Exposures: New users of ARB vs ACEI during the first 12 months of trial follow-up.
Main outcomes and measures: Composite of adjudicated amnestic MCI or PD.
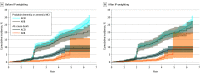
Results: Of 9361 participants, 727 and 1313 new users of an ARB or ACEI, respectively, with well-balanced baseline characteristics between medication exposure groups after inverse probability weighting (mean [SD] age, 67 [9.5] years; 1291 ]63%] male; 240 [33%] Black; 89 [12%] Hispanic; 383 [53%] White; and 15 [2%] other race or ethnicity. In the primary analysis, during a median follow-up of 4.9 years, the inverse probability-weighted rate of amnestic MCI or PD was 4.3 vs 4.6 per 100 person-years among participants initiating ARB vs ACEI (HR, 0.93; 95% CI, 0.76-1.13). In subgroup analyses, new users of an ARB vs ACEI had a lower rate of amnestic MCI or PD among those in the standard systolic blood pressure treatment arm (HR, 0.61; 95% CI, 0.41-0.91) but not in the intensive arm (HR, 1.17; 95% CI, 0.90-1.52) (P = .007 for interaction).
Conclusions and relevance: In this observational cohort study of US adults at high cardiovascular disease risk, there was no difference in the rate of amnestic MCI or PD among new users of an ARB compared with ACEI, although 95% CIs were wide.
Conflict of interest statement
Figures

References
-
- Whelton PK, Carey RM, Aronow WS, et al. . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

