Choline supplementation attenuates experimental sepsis-associated acute kidney injury
- PMID: 35834274
- PMCID: PMC9394731
- DOI: 10.1152/ajprenal.00033.2022
Choline supplementation attenuates experimental sepsis-associated acute kidney injury
Abstract
Acute kidney injury (AKI) is common in critically ill patients, and sepsis is its leading cause. Sepsis-associated AKI (SA-AKI) causes greater morbidity and mortality than other AKI etiologies, yet the underlying mechanisms are incompletely understood. Metabolomic technologies can characterize cellular energy derangements, but few discovery analyses have evaluated the metabolomic profile of SA-AKI. To identify metabolic derangements amenable to therapeutic intervention, we assessed plasma and urine metabolites in septic mice and critically ill children and compared them by AKI status. Metabolites related to choline and central carbon metabolism were differentially abundant in SA-AKI in both mice and humans. Gene expression of enzymes related to choline metabolism was altered in the kidneys and liver of mice with SA-AKI. Treatment with intraperitoneal choline improved renal function in septic mice. Because pediatric patients with sepsis displayed similar metabolomic profiles to septic mice, choline supplementation may attenuate pediatric septic AKI.NEW & NOTEWORTHY Altered choline metabolism plays a role in both human and murine sepsis-associated acute kidney injury (SA-AKI), and choline administration in experimental SA-AKI improved renal function. These findings indicate that 1) mouse models can help interrogate clinically relevant mechanisms and 2) choline supplementation may ameliorate human SA-AKI. Future research will investigate clinically the impact of choline supplementation on human renal function in sepsis and, using model systems, how choline mediates its effects.
Keywords: acute kidney injury; central carbon metabolism; choline; metabolomics; sepsis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


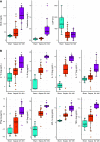


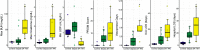

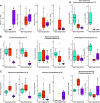

References
-
- Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA. ; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol 2: 431–439, 2007. doi: 10.2215/CJN.03681106. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

