Ustekinumab treatment in ulcerative colitis: Real-world data from the Swedish inflammatory bowel disease quality register
- PMID: 35834389
- PMCID: PMC9486503
- DOI: 10.1002/ueg2.12275
Ustekinumab treatment in ulcerative colitis: Real-world data from the Swedish inflammatory bowel disease quality register
Abstract
Background: Real-world data on clinical outcomes of ustekinumab in ulcerative colitis are lacking.
Objective: To assess short- and long-term clinical outcomes of ustekinumab in ulcerative colitis.
Methods: Adult ulcerative colitis patients without previous colectomy starting ustekinumab treatment up until 11 December 2020 were identified through the Swedish Inflammatory Bowel Disease Register (SWIBREG). Prospectively recorded data were extracted from the SWIBREG. The primary outcome was persistence to ustekinumab 16 weeks after treatment initiation. Secondary outcomes included drug persistence beyond week 16, clinical remission (defined as a patient-reported Mayo rectal bleeding subscore = 0 and stool frequency subscore ≤1), biochemical remission (defined as faecal-calprotectin <250 μg/g) and changes in health-related quality of life (HRQoL), as measured by the Short Health Scale (SHS). Logistic regression was used to identify potential predictors of ustekinumab persistence at 16 weeks.
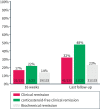
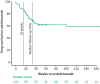
Results: Of the 133 patients with ulcerative colitis, only three were naïve to biologics and tofacitinib. The persistence rates of ustekinumab were 115/133 (86%) at 16 weeks and 89/133 (67%) at last follow-up, that is, after a median follow-up of 32 (interquartile range 19-56) weeks. The clinical remission rates were 17% at 16 weeks and 32% at the last follow-up. The corresponding rates for biochemical remission were 14% and 23%. The median faecal-calprotectin concentration decreased from 740 μg/g at baseline to 98 μg/g at the last follow-up (p < 0.01, n = 37). Improvement was seen in each dimension of the SHS between baseline and last follow-up (p < 0.01 for each dimension, n = 46). Male sex was associated with ustekinumab persistence at 16 weeks (adjusted odds ratio = 4.00, 95% confidence interval: 1.35-11.83).
Conclusion: In this nationwide real-world cohort of ulcerative colitis patients with prior drug failures, including other biologics and tofacitinib, ustekinumab was associated with high drug persistence rates and improvements in clinical, biochemical and HRQoL measures.
Keywords: anti-tnf-antibodies; inflammatory bowel disease; ulcerative colitis; ustekinumab.
© 2022 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
Joel Thunberg has nothing to declare. Olle Björkqvist has nothing to declare. Charlotte R. H. Hedin has received speaker fees from Takeda, Ferring, AbbVie, and Janssen, and consultancy fees from Pfizer. She has acted as local principal investigator for clinical trials for Janssen and GlaxoSmithKline. She is PI on projects at the Karolinska Institutet partly by investigator‐initiated grants from Takeda and Tillotts. None of these activities have any relation to the present study. Anders Forss has served as a speaker for Janssen. Charlotte Söderman has nothing to declare. Daniel Bergemalm reports personal fees from Ferring, Takeda, Janssen and BMS outside the submitted work. Jonas F Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). That study has received funding from the Janssen corporation. Ola Olén has been PI on projects at Karolinska Institutet partly financed by investigator‐initiated grants from Janssen and Ferring and reported a grant from Pfizer in a national safety monitoring programme. None of these studies have any relation to the present study. Karolinska Institutet has received fees for lectures and participation on advisory boards by OO from Janssen, Ferring, Takeda and Pfizer on topics not related to the present study. Henrik Hjortswang has served on the advisory board to AbbVie, Fresenius Kabi, Janssen, Norgine, Pfizer, Takeda and Tillotts. Henrik Hjortswang has served as a speaker for AbbVie, Janssen, Takeda and Tillotts. Hans Strid has served as a speaker or as an advisory board member for AbbVie, Ferring, Janssen, Pfizer, Takeda, Gilead and Tillotts Pharma. Carl Eriksson received grant support/lecture fee/advisory board from Takeda, Janssen Cilag, Pfizer and AbbVie. Jonas Halfvarson has served as a speaker, consultant or advisory board member: AbbVie, Aqilion, Celgene, Celltrion, Dr Falk Pharma and the Falk Foundation, Ferring, Galapagos, Gilead, Index Pharma, Janssen, Lincs, MSD, Novartis, Olink Proteomics, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, and received grant support from Janssen, MSD and Takeda.
Figures
Comment in
-
Ustekinumab in ulcerative colitis- insights from the real-world data.United European Gastroenterol J. 2022 Sep;10(7):621-622. doi: 10.1002/ueg2.12278. Epub 2022 Jul 15. United European Gastroenterol J. 2022. PMID: 35841129 Free PMC article. No abstract available.
References
-
- Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology : Off. J Am Gastroenterol Assoc. 2015;148(5):1035–58. 10.1053/j.gastro.2015.03.001 - DOI - PubMed