Safety Monitoring of COVID-19 mRNA Vaccine First Booster Doses Among Persons Aged ≥12 Years with Presumed Immunocompromise Status - United States, January 12, 2022-March 28, 2022
- PMID: 35834416
- PMCID: PMC9290389
- DOI: 10.15585/mmwr.mm7128a3
Safety Monitoring of COVID-19 mRNA Vaccine First Booster Doses Among Persons Aged ≥12 Years with Presumed Immunocompromise Status - United States, January 12, 2022-March 28, 2022
Abstract
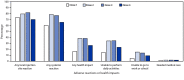
Persons with moderate to severe immunocompromising conditions are at risk for severe COVID-19, and their immune response to COVID-19 vaccination might not be as robust as the response in persons who are not immunocompromised* (1). The Advisory Committee on Immunization Practices (ACIP) recommends that immunocompromised persons aged ≥12 years complete a 3-dose primary mRNA COVID-19 vaccination series followed by a first booster dose (dose 4) ≥3 months after dose 3 and a second booster dose (dose 5) ≥4 months after dose 4.† To characterize the safety of first booster doses among immunocompromised persons aged ≥12 years during January 12, 2022-March 28, 2022, CDC reviewed adverse events and health impact assessments reported to v-safe and the Vaccine Adverse Event Reporting System (VAERS) during the week after receipt of an mRNA COVID-19 first booster dose. V-safe is a voluntary smartphone-based safety surveillance system for adverse events after COVID-19 vaccination. VAERS is a passive surveillance system for all vaccine-associated adverse events co-managed by CDC and the Food and Drug Administration (FDA). A fourth mRNA dose reported to v-safe or VAERS during January 12, 2022-March 28, 2022, was presumed to be an mRNA COVID-19 vaccine booster dose administered to an immunocompromised person because no other population was authorized to receive a fourth dose during that period (2,3). In the United States, during January 12, 2022-March 28, 2022, approximately 518,113 persons aged ≥12 years received a fourth dose. Among 4,015 v-safe registrants who received a fourth dose, local and systemic reactions were less frequently reported than were those following dose 3 of their primary series. VAERS received 145 reports after fourth doses; 128 (88.3%) were nonserious and 17 (11.7%) were serious. Health care providers, immunocompromised persons, and parents of immunocompromised children should be aware that local and systemic reactions are expected after a first booster mRNA COVID-19 vaccine dose, serious adverse events are rare, and safety findings were consistent with those previously described among nonimmunocompromised persons (4,5).
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Safety Monitoring of COVID-19 mRNA Vaccine Second Booster Doses Among Adults Aged ≥50 Years - United States, March 29, 2022-July 10, 2022.MMWR Morb Mortal Wkly Rep. 2022 Jul 29;71(30):971-976. doi: 10.15585/mmwr.mm7130a4. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35900925 Free PMC article.
-
Safety Monitoring of Bivalent COVID-19 mRNA Vaccine Booster Doses Among Children Aged 5-11 Years - United States, October 12-January 1, 2023.MMWR Morb Mortal Wkly Rep. 2023 Jan 13;72(2):39-43. doi: 10.15585/mmwr.mm7202a5. MMWR Morb Mortal Wkly Rep. 2023. PMID: 36634021 Free PMC article.
-
Safety Monitoring of COVID-19 Vaccine Booster Doses Among Adults - United States, September 22, 2021-February 6, 2022.MMWR Morb Mortal Wkly Rep. 2022 Feb 18;71(7):249-254. doi: 10.15585/mmwr.mm7107e1. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35176008 Free PMC article.
-
Safety of co-administration of mRNA COVID-19 and seasonal inactivated influenza vaccines in the vaccine adverse event reporting system (VAERS) during July 1, 2021-June 30, 2022.Vaccine. 2023 Mar 10;41(11):1859-1863. doi: 10.1016/j.vaccine.2022.12.069. Epub 2023 Jan 9. Vaccine. 2023. PMID: 36669964 Free PMC article. Review.
-
Post-authorization safety surveillance of Ad.26.COV2.S vaccine: Reports to the Vaccine Adverse Event Reporting System and v-safe, February 2021-February 2022.Vaccine. 2023 Jul 5;41(30):4422-4430. doi: 10.1016/j.vaccine.2023.06.023. Epub 2023 Jun 14. Vaccine. 2023. PMID: 37321898 Free PMC article. Review.
Cited by
-
Association Between the "We Can Do This" Campaign and COVID-19 Booster Uptake, U.S., 2021-2022.AJPM Focus. 2024 Jan 4;3(2):100183. doi: 10.1016/j.focus.2024.100183. eCollection 2024 Apr. AJPM Focus. 2024. PMID: 38357552 Free PMC article.
-
Autoimmune/inflammatory syndrome induced by adjuvants (ASIA): past, present, and future implications.Clin Exp Immunol. 2023 Jul 5;213(1):87-101. doi: 10.1093/cei/uxad033. Clin Exp Immunol. 2023. PMID: 36881788 Free PMC article. Review.
-
COVID-19 Vaccination Recommendations for Immunocompromised Patient Populations: Delphi Panel and Consensus Statement Generation in the United States.Infect Dis Ther. 2024 Nov;13(11):2255-2283. doi: 10.1007/s40121-024-01052-8. Epub 2024 Oct 10. Infect Dis Ther. 2024. PMID: 39387989 Free PMC article.
-
Vaccines and Autoimmunity-From Side Effects to ASIA Syndrome.Medicina (Kaunas). 2023 Feb 14;59(2):364. doi: 10.3390/medicina59020364. Medicina (Kaunas). 2023. PMID: 36837564 Free PMC article. Review.
References
-
- Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine letter of authorization (reissued). Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2022. https://www.fda.gov/media/150386/download
-
- Food and Drug Administration. Moderna COVID-19 vaccine letter of authorization (reissued). Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. https://www.fda.gov/media/144636/download
- doi: 10.1016/j.vaccine.2015.07.035. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

