Fungal-mediated lung allergic airway disease: The critical role of macrophages and dendritic cells
- PMID: 35834490
- PMCID: PMC9282651
- DOI: 10.1371/journal.ppat.1010608
Fungal-mediated lung allergic airway disease: The critical role of macrophages and dendritic cells
Abstract
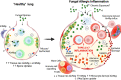
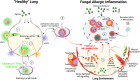
Fungi are abundant in the environment, causing our lungs to be constantly exposed to a diverse range of species. While the majority of these are cleared effectively in healthy individuals, constant exposure to spores (especially Aspergillus spp.) can lead to the development of allergic inflammation that underpins and worsen diseases such as asthma. Despite this, the precise mechanisms that underpin the development of fungal allergic disease are poorly understood. Innate immune cells, such as macrophages (MΦs) and dendritic cells (DCs), have been shown to be critical for mediating allergic inflammation to a range of different allergens. This review will focus on the crucial role of MΦ and DCs in mediating antifungal immunity, evaluating how these immune cells mediate allergic inflammation within the context of the lung environment. Ultimately, we aim to highlight important future research questions that will lead to novel therapeutic strategies for fungal allergic diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Mgl2+ cDC2s coordinate fungal allergic airway type 2, but not type 17, inflammation in mice.Nat Commun. 2025 Jan 22;16(1):928. doi: 10.1038/s41467-024-55663-3. Nat Commun. 2025. PMID: 39843887 Free PMC article.
-
Dimethyl fumarate abrogates dust mite-induced allergic asthma by altering dendritic cell function.Immun Inflamm Dis. 2019 Sep;7(3):201-213. doi: 10.1002/iid3.262. Epub 2019 Jul 2. Immun Inflamm Dis. 2019. PMID: 31264384 Free PMC article.
-
Allergic Fungal Airway Disease.J Investig Allergol Clin Immunol. 2016;26(6):344-354. doi: 10.18176/jiaci.0122. J Investig Allergol Clin Immunol. 2016. PMID: 27996940 Review.
-
Fibrinogen cleavage products and Toll-like receptor 4 promote the generation of programmed cell death 1 ligand 2-positive dendritic cells in allergic asthma.J Allergy Clin Immunol. 2018 Aug;142(2):530-541.e6. doi: 10.1016/j.jaci.2017.09.019. Epub 2017 Oct 14. J Allergy Clin Immunol. 2018. PMID: 29038008
-
The role of dendritic and epithelial cells as master regulators of allergic airway inflammation.Lancet. 2010 Sep 4;376(9743):835-43. doi: 10.1016/S0140-6736(10)61226-3. Lancet. 2010. PMID: 20816550 Review.
Cited by
-
Refractory phenotype of Aspergillus-sensitized asthma with bronchiectasis and allergic bronchopulmonary aspergillosis.J Allergy Clin Immunol Glob. 2024 Nov 1;4(1):100364. doi: 10.1016/j.jacig.2024.100364. eCollection 2025 Feb. J Allergy Clin Immunol Glob. 2024. PMID: 39659740 Free PMC article.
-
Pathogenicity and virulence of Aspergillus fumigatus.Virulence. 2023 Dec;14(1):2172264. doi: 10.1080/21505594.2023.2172264. Virulence. 2023. PMID: 36752587 Free PMC article. Review.
-
A scoping review: What are the cellular mechanisms that drive the allergic inflammatory response to fungal allergens in the lung epithelium?Clin Transl Allergy. 2023 Jun;13(6):e12252. doi: 10.1002/clt2.12252. Clin Transl Allergy. 2023. PMID: 37357550 Free PMC article.
-
Tolerogenic dendritic cells in radiation-induced lung injury.Front Immunol. 2024 Jan 8;14:1323676. doi: 10.3389/fimmu.2023.1323676. eCollection 2023. Front Immunol. 2024. PMID: 38259434 Free PMC article. Review.
-
More than Just Protein Degradation: The Regulatory Roles and Moonlighting Functions of Extracellular Proteases Produced by Fungi Pathogenic for Humans.J Fungi (Basel). 2023 Jan 15;9(1):121. doi: 10.3390/jof9010121. J Fungi (Basel). 2023. PMID: 36675942 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

