Human immune globulin treatment controls Zika viremia in pregnant rhesus macaques
- PMID: 35834540
- PMCID: PMC9282477
- DOI: 10.1371/journal.pone.0266664
Human immune globulin treatment controls Zika viremia in pregnant rhesus macaques
Abstract
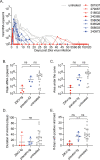
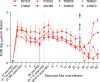
There are currently no approved drugs to treat Zika virus (ZIKV) infection during pregnancy. Hyperimmune globulin products such as VARIZIG and WinRho are FDA-approved to treat conditions during pregnancy such as Varicella Zoster virus infection and Rh-incompatibility. We administered ZIKV-specific human immune globulin as a treatment in pregnant rhesus macaques one day after subcutaneous ZIKV infection. All animals controlled ZIKV viremia following the treatment and generated robust levels of anti-Zika virus antibodies in their blood. No adverse fetal or infant outcomes were identified in the treated animals, yet the placebo control treated animals also did not have signs related to congenital Zika syndrome (CZS). Human immune globulin may be a viable prophylaxis and treatment option for ZIKV infection during pregnancy, however, more studies are required to fully assess the impact of this treatment to prevent CZS.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: DHO is a paid consultant for Battelle, devoted to research in the areas of assisting in the design and interpretation of their nonhuman primate ZIKV studies. His relationship does not carry with it any restrictions on publication, and any associated intellectual property will be disclosed and processed according to UW-Madison policy. None of the animals used in this study are involved in any studies with Battelle. The publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The following patents have been filed that pertain to the results in this manuscript: PCT/IB2019/055275 and PCT/IB2019/053463.
Figures



References
-
- Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, et al. Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure—U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep. 2017;66: 366–373. doi: 10.15585/mmwr.mm6613e1 - DOI - PMC - PubMed
-
- World Health Organization. Zika epidemiology update. In: World Health Organization Emergencies [Internet]. 2 Jul 2019. [cited 24 Mar 2021]. Available: https://www.who.int/emergencies/diseases/zika/epidemiology-update/en/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

