Longitudinal Monitoring of Circulating Tumor DNA to Predict Treatment Outcomes in Advanced Cancers
- PMID: 35834760
- PMCID: PMC9307306
- DOI: 10.1200/PO.21.00512
Longitudinal Monitoring of Circulating Tumor DNA to Predict Treatment Outcomes in Advanced Cancers
Abstract
Purpose: The response to cancer therapies is typically assessed with radiologic imaging 6-10 weeks after treatment initiation. Circulating tumor DNA (ctDNA), however, has a short half-life, and dynamic changes in ctDNA quantity may allow for earlier assessment of the therapeutic response.
Methods: Patients with advanced solid tumors referred to the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center were invited to participate in a liquid biopsy protocol for which serial blood samples were collected before, during, and after systemic therapy. We isolated ctDNA from serially collected plasma samples at baseline, mid-treatment, and first restaging. Genomically informed droplet digital polymerase chain reaction (ddPCR) was performed, and ctDNA quantities were reported as aggregate variant allele frequencies for all detected molecular aberrations.
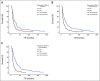
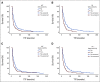
Results: We included 204 patients receiving 260 systemic therapies. The ctDNA detection rate was higher in progressors (patients with progressive disease) compared with nonprogressors (patients with stable disease, partial responses, or complete responses) at all time points (P < .009). Moreover, ctDNA detection was associated with a shorter median time-to-treatment failure (P ≤ .001). Positive delta and slope values for changes in ctDNA quantity were more frequent in progressors (P ≤ .03 and P < .001, respectively) and were associated with a shorter median time-to-treatment failure (P ≤ .014 and P < .001, respectively). Increasing ctDNA quantity was predictive of clinical and/or radiologic progressive disease in 73% of patients (median lead time, 23 days).
Conclusion: Detection of ctDNA and early dynamic changes in its quantity can predict the clinical outcomes of systemic therapies in patients with advanced solid tumors.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Lim JSJ, Janku F, Yap TA.Circulating tumor DNA-From bench to bedside Curr Probl Cancer 41212–2212017 - PubMed
-
- Rolfo C, Mack P, Scagliotti GV, et al. Liquid biopsy for advanced NSCLC: A consensus statement from the International Association for the Study of Lung Cancer Res Social Adm Pharm 161647–16622021 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

