Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer
- PMID: 35834777
- PMCID: PMC10476841
- DOI: 10.1200/JCO.21.01604
Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer
Erratum in
-
Erratum: Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer.J Clin Oncol. 2024 Jun 10;42(17):2112. doi: 10.1200/JCO.24.00821. Epub 2024 Apr 30. J Clin Oncol. 2024. PMID: 38687918 No abstract available.
Abstract
Purpose: The phase III POLO study demonstrated significant progression-free survival (PFS) benefit for active olaparib maintenance therapy versus placebo for patients with metastatic pancreatic adenocarcinoma and a germline BRCA mutation. Here, we report the final analysis of overall survival (OS) and other secondary end points.
Patients and methods: Patients with a deleterious or suspected deleterious germline BRCA mutation whose disease had not progressed after ≥ 16 weeks of first-line platinum-based chemotherapy were randomly assigned 3:2 to active maintenance olaparib (300 mg twice daily) or placebo. The primary end point was PFS; secondary end points included OS, time to second disease progression or death, time to first and second subsequent cancer therapies or death, time to discontinuation of study treatment or death, and safety and tolerability.
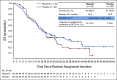
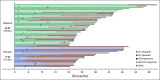
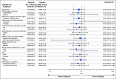
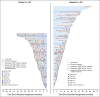
Results: In total, 154 patients were randomly assigned (olaparib, n = 92; placebo, n = 62). No statistically significant OS benefit was observed (median 19.0 v 19.2 months; hazard ratio [HR], 0.83; 95% CI, 0.56 to 1.22; P = .3487). Kaplan-Meier OS curves separated at approximately 24 months, and the estimated 3-year survival after random assignment was 33.9% versus 17.8%, respectively. Median time to first subsequent cancer therapy or death (HR, 0.44; 95% CI, 0.30 to 0.66; P < .0001), time to second subsequent cancer therapy or death (HR, 0.61; 95% CI, 0.42 to 0.89; P = .0111), and time to discontinuation of study treatment or death (HR, 0.43; 95% CI, 0.29 to 0.63; P < .0001) significantly favored olaparib. The HR for second disease progression or death favored olaparib without reaching statistical significance (HR, 0.66; 95% CI, 0.43 to 1.02; P = .0613). Olaparib was well tolerated with no new safety signals.
Conclusion: Although no statistically significant OS benefit was observed, the HR numerically favored olaparib, which also conferred clinically meaningful benefits including increased time off chemotherapy and long-term survival in a subset of patients.
Trial registration: ClinicalTrials.gov NCT02184195.
Conflict of interest statement
Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



References
-
- Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 - PubMed
-
- GBD 2017 Pancreatic Cancer Collaborators : The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 4:934-947, 2019 - PMC - PubMed
-
- Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 - PubMed
-
- Ducreux M, Cuhna AS, Caramella C, et al. : Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v56-68, 2015. (suppl 5) - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

