Pre-clinical evaluation of antiviral activity of nitazoxanide against SARS-CoV-2
- PMID: 35834886
- PMCID: PMC9271885
- DOI: 10.1016/j.ebiom.2022.104148
Pre-clinical evaluation of antiviral activity of nitazoxanide against SARS-CoV-2
Abstract
Background: To address the emergence of SARS-CoV-2, multiple clinical trials in humans were rapidly started, including those involving an oral treatment by nitazoxanide, despite no or limited pre-clinical evidence of antiviral efficacy.
Methods: In this work, we present a complete pre-clinical evaluation of the antiviral activity of nitazoxanide against SARS-CoV-2.
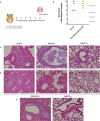
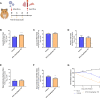
Findings: First, we confirmed the in vitro efficacy of nitazoxanide and tizoxanide (its active metabolite) against SARS-CoV-2. Then, we demonstrated nitazoxanide activity in a reconstructed bronchial human airway epithelium model. In a SARS-CoV-2 virus challenge model in hamsters, oral and intranasal treatment with nitazoxanide failed to impair viral replication in commonly affected organs. We hypothesized that this could be due to insufficient diffusion of the drug into organs of interest. Indeed, our pharmacokinetic study confirmed that concentrations of tizoxanide in organs of interest were always below the in vitro EC50.
Interpretation: These preclinical results suggest, if directly applicable to humans, that the standard formulation and dosage of nitazoxanide is not effective in providing antiviral therapy for Covid-19.
Funding: This work was supported by the Fondation de France "call FLASH COVID-19", project TAMAC, by "Institut national de la santé et de la recherche médicale" through the REACTing (REsearch and ACTion targeting emerging infectious diseases), by REACTING/ANRS MIE under the agreement No. 21180 ('Activité des molécules antivirales dans le modèle hamster'), by European Virus Archive Global (EVA 213 GLOBAL) funded by the European Union's Horizon 2020 research and innovation program under grant agreement No. 871029 and DNDi under support by the Wellcome Trust Grant ref: 222489/Z/21/Z through the COVID-19 Therapeutics Accelerator".
Keywords: Animal model; Antiviral therapy; COVID-19; Nitazoxanide; Pre-clinical research; SARS-CoV-2.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests None to declare.
Figures




References
-
- WHO. World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. - PubMed
-
- Kratky M, Vinsova J. Antiviral activity of substituted salicylanilides–a review. Mini Rev Med Chem. 2011;11(11):956–967. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

