A multiple-target mRNA-LNP vaccine induces protective immunity against experimental multi-serotype DENV in mice
- PMID: 35835315
- PMCID: PMC9583182
- DOI: 10.1016/j.virs.2022.07.003
A multiple-target mRNA-LNP vaccine induces protective immunity against experimental multi-serotype DENV in mice
Abstract
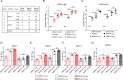
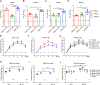
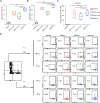
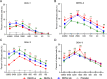
Dengue virus (DENV) is a mosquito-borne virus with a rapid spread to humans, causing mild to potentially fatal illness in hundreds of millions of people each year. Due to the large number of serotypes of the virus, there remains an unmet need to develop protective vaccines for a broad spectrum of the virus. Here, we constructed a modified mRNA vaccine containing envelope domain III (E-DIII) and non-structural protein 1 (NS1) coated with lipid nanoparticles. This multi-target vaccine induced a robust antiviral immune response and increased neutralizing antibody titers that blocked all four types of DENV infection in vitro without significant antibody-dependent enhancement (ADE). In addition, there was more bias for Th1 than Th2 in the exact E-DIII and NS1-specific T cell responses after a single injection. Importantly, intramuscular immunization limited DENV transmission in vivo and eliminated vascular leakage. Our findings highlight that chimeric allogeneic structural and non-structural proteins can be effective targets for DENV vaccine and that they can prevent the further development of congenital DENV syndrome.
Keywords: Dengue virus (DENV); Envelope domain III (E-DIII); Immune response; Multi-serotype; Non-structural protein 1 (NS1); mRNA vaccine.
Copyright © 2022 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
A Dengue Virus Serotype 1 mRNA-LNP Vaccine Elicits Protective Immune Responses.J Virol. 2021 May 24;95(12):e02482-20. doi: 10.1128/JVI.02482-20. Print 2021 May 24. J Virol. 2021. PMID: 33762420 Free PMC article.
-
Enhanced immunogenicity of an mRNA vaccine against dengue virus serotype 2 with modified key residue.Vaccine. 2025 May 31;57:127216. doi: 10.1016/j.vaccine.2025.127216. Epub 2025 May 14. Vaccine. 2025. PMID: 40373693
-
Universal Dengue Vaccine Elicits Neutralizing Antibodies against Strains from All Four Dengue Virus Serotypes.J Virol. 2021 Jan 28;95(4):e00658-20. doi: 10.1128/JVI.00658-20. Print 2021 Jan 28. J Virol. 2021. PMID: 33208445 Free PMC article.
-
Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine.Front Cell Infect Microbiol. 2020 Oct 22;10:572681. doi: 10.3389/fcimb.2020.572681. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194810 Free PMC article. Review.
-
A recombinant, chimeric tetravalent dengue vaccine candidate based on a dengue virus serotype 2 backbone.Expert Rev Vaccines. 2016;15(4):497-508. doi: 10.1586/14760584.2016.1128328. Epub 2016 Feb 22. Expert Rev Vaccines. 2016. PMID: 26635182 Review.
Cited by
-
Development of New Live-Attenuated Vaccine Candidates Lacking Antibody-Dependent Enhancement (ADE) Against Dengue.Vaccines (Basel). 2025 May 16;13(5):532. doi: 10.3390/vaccines13050532. Vaccines (Basel). 2025. PMID: 40432141 Free PMC article. Review.
-
Revolutionizing immunization: a comprehensive review of mRNA vaccine technology and applications.Virol J. 2025 Mar 12;22(1):71. doi: 10.1186/s12985-025-02645-6. Virol J. 2025. PMID: 40075519 Free PMC article. Review.
-
Multitarget Compounds for Neglected Diseases: A Review.Curr Drug Targets. 2024;25(9):577-601. doi: 10.2174/0113894501298864240627060247. Curr Drug Targets. 2024. PMID: 38967077 Review.
-
Research progress of mosquito-borne virus mRNA vaccines.Mol Ther Methods Clin Dev. 2024 Dec 12;33(1):101398. doi: 10.1016/j.omtm.2024.101398. eCollection 2025 Mar 13. Mol Ther Methods Clin Dev. 2024. PMID: 39834558 Free PMC article. Review.
-
mRNA Vaccine Development in the Fight Against Zoonotic Viral Diseases.Viruses. 2025 Jul 8;17(7):960. doi: 10.3390/v17070960. Viruses. 2025. PMID: 40733577 Free PMC article. Review.
References
-
- Beatty P.R., Puerta-Guardo H., Killingbeck S.S., Glasner D.R., Hopkins K., Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 2015;7:304ra141. - PubMed
-
- Bhamarapravati N., Sutee Y. Live attenuated tetravalent dengue vaccine. Vaccine. 2000;18:44–47. - PubMed
-
- Biswal S., Reynales H., Saez-Llorens X., Lopez P., Borja-Tabora C., Kosalaraksa P., Sirivichayakul C., Watanaveeradej V., Rivera L., Espinoza F., Fernando L., Dietze R., Luz K., Venancio Da Cunha R., Jimeno J., Lopez-Medina E., Borkowski A., Brose M., Rauscher M., Lefevre I., Bizjajeva S., Bravo L., Wallace D., Group T.S. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N. Engl. J. Med. 2019;381:2009–2019. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

