Channel Function of Polycystin-2 in the Endoplasmic Reticulum Protects against Autosomal Dominant Polycystic Kidney Disease
- PMID: 35835458
- PMCID: PMC9342640
- DOI: 10.1681/ASN.2022010053
Channel Function of Polycystin-2 in the Endoplasmic Reticulum Protects against Autosomal Dominant Polycystic Kidney Disease
Abstract
Background: Mutations of PKD2, which encodes polycystin-2, cause autosomal dominant polycystic kidney disease (ADPKD). The prevailing view is that defects in polycystin-2-mediated calcium ion influx in the primary cilia play a central role in the pathogenesis of cyst growth. However, polycystin-2 is predominantly expressed in the endoplasmic reticulum (ER) and more permeable to potassium ions than to calcium ions.
Methods: The trimeric intracellular cation (TRIC) channel TRIC-B is an ER-resident potassium channel that mediates potassium-calcium counterion exchange for inositol trisphosphate-mediated calcium ion release. Using TRIC-B as a tool, we examined the function of ER-localized polycystin-2 and its role in ADPKD pathogenesis in cultured cells, zebrafish, and mouse models.
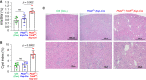
Results: Agonist-induced ER calcium ion release was defective in cells lacking polycystin-2 and reversed by exogenous expression of TRIC-B. Vice versa, exogenous polycystin-2 reversed an ER calcium-release defect in cells lacking TRIC-B. In a zebrafish model, expression of wild-type but not nonfunctional TRIC-B suppressed polycystin-2-deficient phenotypes. Similarly, these phenotypes were suppressed by targeting the ROMK potassium channel (normally expressed on the cell surface) to the ER. In cultured cells and polycystin-2-deficient zebrafish phenotypes, polycystin-2 remained capable of reversing the ER calcium release defect even when it was not present in the cilia. Transgenic expression of Tric-b ameliorated cystogenesis in the kidneys of conditional Pkd2-inactivated mice, whereas Tric-b deletion enhanced cystogenesis in Pkd2-heterozygous kidneys.
Conclusions: Polycystin-2 in the ER appears to be critical for anticystogenesis and likely functions as a potassium ion channel to facilitate potassium-calcium counterion exchange for inositol trisphosphate-mediated calcium release. The results advance the understanding of ADPKD pathogenesis and provides proof of principle for pharmacotherapy by TRIC-B activators.
Keywords: ADPKD; endoplasmic reticulum; ion channel; polycystin.
Copyright © 2022 by the American Society of Nephrology.
Figures








Comment in
-
Polycystin-2 in the Endoplasmic Reticulum: Bending Ideas about the Role of the Cilium.J Am Soc Nephrol. 2022 Aug;33(8):1433-1434. doi: 10.1681/ASN.2022050557. J Am Soc Nephrol. 2022. PMID: 35906088 Free PMC article. No abstract available.
References
-
- Igarashi P, Somlo S: Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13: 2384–2398, 2002 - PubMed
-
- Watnick TJ, Torres VE, Gandolph MA, Qian F, Onuchic LF, Klinger KW, et al. : Somatic mutation in individual liver cysts supports a two-hit model of cystogenesis in autosomal dominant polycystic kidney disease. Mol Cell 2: 247–251, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

