Phagocytosis of Plasmodium falciparum ring-stage parasites predicts protection against malaria
- PMID: 35835738
- PMCID: PMC9281573
- DOI: 10.1038/s41467-022-31640-6
Phagocytosis of Plasmodium falciparum ring-stage parasites predicts protection against malaria
Abstract
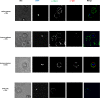
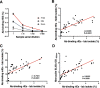
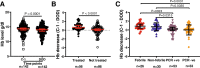
Ring-infected erythrocytes are the predominant asexual stage in the peripheral circulation but are rarely investigated in the context of acquired immunity against Plasmodium falciparum malaria. Here we compare antibody-dependent phagocytosis of ring-infected parasite cultures in samples from a controlled human malaria infection (CHMI) study (NCT02739763). Protected volunteers did not develop clinical symptoms, maintained parasitaemia below a predefined threshold of 500 parasites/μl and were not treated until the end of the study. Antibody-dependent phagocytosis of both ring-infected and uninfected erythrocytes from parasite cultures was strongly correlated with protection. A surface proteomic analysis revealed the presence of merozoite proteins including erythrocyte binding antigen-175 and -140 on ring-infected and uninfected erythrocytes, providing an additional antibody-mediated protective mechanism for their activity beyond invasion-inhibition. Competition phagocytosis assays support the hypothesis that merozoite antigens are the key mediators of this functional activity. Targeting ring-stage parasites may contribute to the control of parasitaemia and prevention of clinical malaria.
© 2022. The Author(s).
Conflict of interest statement
B.K.L.S., Y.A., P.F.B., S.L.H, E.R.J., TR. are salaried, full-time employees of Sanaria Inc., the manufacturer of Sanaria PfSPZ Challenge. Thus, all authors associated with Sanaria Inc. have potential conflicts of interest. All other authors declare no competing interests.
Figures



Comment in
-
Housebreaking Plasmodium parasites leave their fingerprints at the door.Trends Parasitol. 2022 Nov;38(11):921-923. doi: 10.1016/j.pt.2022.09.005. Epub 2022 Sep 23. Trends Parasitol. 2022. PMID: 36163104
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

