Chromosomal phase improves aneuploidy detection in non-invasive prenatal testing at low fetal DNA fractions
- PMID: 35835769
- PMCID: PMC9283487
- DOI: 10.1038/s41598-022-14049-5
Chromosomal phase improves aneuploidy detection in non-invasive prenatal testing at low fetal DNA fractions
Abstract
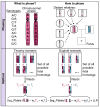
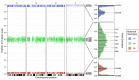
Non-invasive prenatal testing (NIPT) to detect fetal aneuploidy by sequencing the cell-free DNA (cfDNA) in maternal plasma is being broadly adopted. To detect fetal aneuploidies from maternal plasma, where fetal DNA is mixed with far-larger amounts of maternal DNA, NIPT requires a minimum fraction of the circulating cfDNA to be of placental origin, a level which is usually attained beginning at 10 weeks gestational age. We present an approach that leverages the arrangement of alleles along homologous chromosomes-also known as chromosomal phase-to make NIPT analyses more conclusive. We validate our approach with in silico simulations, then re-analyze data from a pregnant mother who, due to a fetal DNA fraction of 3.4%, received an inconclusive aneuploidy determination through NIPT. We find that the presence of a trisomy 18 fetus can be conclusively inferred from the patient's same molecular data when chromosomal phase is incorporated into the analysis. Key to the effectiveness of our approach is the ability of homologous chromosomes to act as natural controls for each other and the ability of chromosomal phase to integrate subtle quantitative signals across very many sequence variants. These results show that chromosomal phase increases the sensitivity of a common laboratory test, an idea that could also advance cfDNA analyses for cancer detection.
© 2022. The Author(s).
Conflict of interest statement
Giulio Genovese, Po-Ru Loh, and Steven A. Mccarroll declare competing interests: patent application PCT/WO2019/079493 has been filed on the mosaic chromosomal alterations detection method used in this work. This does not restrict the non-commercial use of the method described in this article. The other authors have no competing interests.
Figures



References
-
- Porreco RP, et al. Noninvasive prenatal screening for fetal trisomies 21, 18, 13 and the common sex chromosome aneuploidies from maternal blood using massively parallel genomic sequencing of DNA. Am. J. Obstet. Gynecol. 2014;211(365):e1–12. - PubMed
-
- Zhang, H. et al. Non-invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146,958 pregnancies. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol.45, 530–538 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

