Persistence of immunity and impact of third dose of inactivated COVID-19 vaccine against emerging variants
- PMID: 35835822
- PMCID: PMC9281359
- DOI: 10.1038/s41598-022-16097-3
Persistence of immunity and impact of third dose of inactivated COVID-19 vaccine against emerging variants
Abstract
This is a comprehensive report on immunogenicity of COVAXIN® booster dose against ancestral and Variants of Concern (VOCs) up to 12 months. It is well known that neutralizing antibodies induced by COVID-19 vaccines wane within 6 months of vaccination leading to questions on the effectiveness of two-dose vaccination against breakthrough infections. Therefore, we assessed the persistence of immunogenicity up to 6 months after a two or three-dose with BBV152 and the safety of a booster dose in an ongoing phase 2, double-blind, randomized controlled trial (ClinicalTrials.gov: NCT04471519). We report persistence of humoral and cell mediated immunity up to 12 months of vaccination, despite decline in the magnitude of antibody titers. Administration of a third dose of BBV152 increased neutralization titers against both homologous (D614G) and heterologous strains (Alpha, Beta, Delta, Delta Plus and Omicron) with a slight increase in B cell memory responses. Thus, seronversion rate remain high in boosted recipients compared to non-booster, even after 6 months, post third dose against variants. No serious adverse events observed, except pain at the injection site, itching and redness. Hence, these results indicate that a booster dose of BBV152 is safe and necessary to ensure persistent immunity to minimize breakthrough infections of COVID-19, due to newly emerging variants.Trial registration: Registered with the Clinical Trials Registry (India) No. CTRI/2021/04/032942, dated 19/04/2021 and on Clinicaltrials.gov: NCT04471519.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
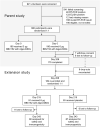
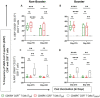
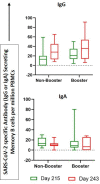

References
-
- Tang, P. et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. 2021.08.11.21261885 (2021) 10.1101/2021.08.11.21261885.
-
- Puranik, A. et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv 2021.08.06.21261707 (2021) 10.1101/2021.08.06.21261707.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

