Molecular basis for the regulation of human glycogen synthase by phosphorylation and glucose-6-phosphate
- PMID: 35835870
- PMCID: PMC9287172
- DOI: 10.1038/s41594-022-00799-3
Molecular basis for the regulation of human glycogen synthase by phosphorylation and glucose-6-phosphate
Abstract
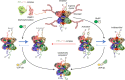
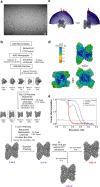
Glycogen synthase (GYS1) is the central enzyme in muscle glycogen biosynthesis. GYS1 activity is inhibited by phosphorylation of its amino (N) and carboxyl (C) termini, which is relieved by allosteric activation of glucose-6-phosphate (Glc6P). We present cryo-EM structures at 3.0-4.0 Å resolution of phosphorylated human GYS1, in complex with a minimal interacting region of glycogenin, in the inhibited, activated and catalytically competent states. Phosphorylations of specific terminal residues are sensed by different arginine clusters, locking the GYS1 tetramer in an inhibited state via intersubunit interactions. The Glc6P activator promotes conformational change by disrupting these interactions and increases the flexibility of GYS1, such that it is poised to adopt a catalytically competent state when the sugar donor UDP-glucose (UDP-glc) binds. We also identify an inhibited-like conformation that has not transitioned into the activated state, in which the locking interaction of phosphorylation with the arginine cluster impedes subsequent conformational changes due to Glc6P binding. Our results address longstanding questions regarding the mechanism of human GYS1 regulation.
© 2022. The Author(s).
Conflict of interest statement
P.M.L., M.T., S.H. and A.B. are employees of Pfizer Inc. T.J.M., L.S., D.S.F., I.M.F. and W.W.Y. declare no competing interests.
Figures














Similar articles
-
Structural basis for glucose-6-phosphate activation of glycogen synthase.Proc Natl Acad Sci U S A. 2010 Oct 12;107(41):17563-8. doi: 10.1073/pnas.1006340107. Epub 2010 Sep 27. Proc Natl Acad Sci U S A. 2010. PMID: 20876143 Free PMC article.
-
Mechanism of glycogen synthase inactivation and interaction with glycogenin.Nat Commun. 2022 Jun 11;13(1):3372. doi: 10.1038/s41467-022-31109-6. Nat Commun. 2022. PMID: 35690592 Free PMC article.
-
A highly prevalent equine glycogen storage disease is explained by constitutive activation of a mutant glycogen synthase.Biochim Biophys Acta Gen Subj. 2017 Jan;1861(1 Pt A):3388-3398. doi: 10.1016/j.bbagen.2016.08.021. Epub 2016 Aug 31. Biochim Biophys Acta Gen Subj. 2017. PMID: 27592162 Free PMC article.
-
Regulation of muscle glycogen synthase phosphorylation and kinetic properties by insulin, exercise, adrenaline and role in insulin resistance.Arch Physiol Biochem. 2009 Feb;115(1):13-21. doi: 10.1080/13813450902778171. Arch Physiol Biochem. 2009. PMID: 19267278 Review.
-
The role of glucose 6-phosphate in the control of glycogen synthase.FASEB J. 1997 Jun;11(7):544-58. FASEB J. 1997. PMID: 9212078 Review.
Cited by
-
Biosynthesis, structure and biological function of cholesterol glucoside in Helicobacter pylori: A review.Medicine (Baltimore). 2023 Sep 8;102(36):e34911. doi: 10.1097/MD.0000000000034911. Medicine (Baltimore). 2023. PMID: 37682174 Free PMC article. Review.
-
Bioactive fraction isolated from Curcuma angustifolia rhizome exerts anti-diabetic effects in vitro, in silico and in vivo by regulating AMPK/PKA signaling pathway.Front Pharmacol. 2025 May 14;16:1570533. doi: 10.3389/fphar.2025.1570533. eCollection 2025. Front Pharmacol. 2025. PMID: 40438603 Free PMC article.
-
Glycogen synthase 1 targeting reveals a metabolic vulnerability in triple-negative breast cancer.J Exp Clin Cancer Res. 2023 Jun 6;42(1):143. doi: 10.1186/s13046-023-02715-z. J Exp Clin Cancer Res. 2023. PMID: 37280675 Free PMC article.
-
Human glycogenins maintain glucose homeostasis by regulating glycogen metabolism.Nat Commun. 2025 Jul 16;16(1):6556. doi: 10.1038/s41467-025-61862-3. Nat Commun. 2025. PMID: 40670355 Free PMC article.
-
Architecture, Function, Regulation, and Evolution of α-Glucans Metabolic Enzymes in Prokaryotes.Chem Rev. 2024 Apr 24;124(8):4863-4934. doi: 10.1021/acs.chemrev.3c00811. Epub 2024 Apr 12. Chem Rev. 2024. PMID: 38606812 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

