Mapping and targeted viral activation of pancreatic nerves in mice reveal their roles in the regulation of glucose metabolism
- PMID: 35835995
- PMCID: PMC9669304
- DOI: 10.1038/s41551-022-00909-y
Mapping and targeted viral activation of pancreatic nerves in mice reveal their roles in the regulation of glucose metabolism
Abstract
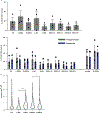
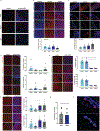
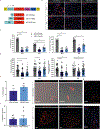
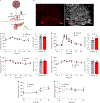
A lack of comprehensive mapping of ganglionic inputs into the pancreas and of technology for the modulation of the activity of specific pancreatic nerves has hindered the study of how they regulate metabolic processes. Here we show that the pancreas-innervating neurons in sympathetic, parasympathetic and sensory ganglia can be mapped in detail by using tissue clearing and retrograde tracing (the tracing of neural connections from the synapse to the cell body), and that genetic payloads can be delivered via intrapancreatic injection to target sites in efferent pancreatic nerves in live mice through optimized adeno-associated viruses and neural-tissue-specific promoters. We also show that, in male mice, the targeted activation of parasympathetic cholinergic intrapancreatic ganglia and neurons doubled plasma-insulin levels and improved glucose tolerance, and that tolerance was impaired by stimulating pancreas-projecting sympathetic neurons. The ability to map the peripheral ganglia innervating the pancreas and to deliver transgenes to specific pancreas-projecting neurons will facilitate the examination of ganglionic inputs and the study of the roles of pancreatic efferent innervation in glucose metabolism.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
S.A.S is a named inventor of the patent ‘Compositions and methods to modulate cell activity’ (US9399063B2). S.A.S. and M.G.K. are co-founders of Redpin Therapeutics, and consult for and have equity in the company. M.G.K. also consults for Meira GTx. All other authors declare no competing interests.
Figures








References
-
- Grapin-Botton A Ductal cells of the pancreas. Int. J. Biochem. Cell Biol. 37, 504–510 (2005). - PubMed
-
- Guenat E et al. Counterregulatory responses to hypoglycemia in patients with glucokinase gene mutations. Diabetes Metab. 26, 377–384 (2000). - PubMed
-
- Thorens B Neural regulation of pancreatic islet cell mass and function. Diabetes Obes. Metab. 16, 87–95 (2014). - PubMed
-
- Yamamoto H, Nagai K & Nakagawa H Bilateral lesions of the SCN abolish lipolytic and hyperphagic responses to 2DG. Physiol. Behav. 32, 1017–1020 (1984). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

