Systematic Review with Meta-Analysis: Efficacy and Safety of Lusutrombopag for Severe Thrombocytopenia in Patients with Chronic Liver Disease Undergoing Invasive Procedures
- PMID: 35836089
- PMCID: PMC9402754
- DOI: 10.1007/s12325-022-02235-w
Systematic Review with Meta-Analysis: Efficacy and Safety of Lusutrombopag for Severe Thrombocytopenia in Patients with Chronic Liver Disease Undergoing Invasive Procedures
Abstract
Introduction: Lusutrombopag is an oral thrombopoietin receptor agonist (TPO-RA). Clinical trials have shown lusutrombopag's efficacy in reducing need for preoperative platelet transfusion in patients with chronic liver disease (CLD) and severe thrombocytopenia. This analysis assessed efficacy and safety of lusutrombopag in patients with severe thrombocytopenia and CLD undergoing planned invasive procedures.
Methods: An electronic database search (through 1 December 2020) identified three randomised, placebo-controlled, double-blind clinical trials comparing lusutrombopag with placebo in patients with CLD and platelet count below 50 × 109/L scheduled to undergo a procedure with a perioperative bleeding risk. A random-effects meta-analysis examined treatment effect, with Cochrane Collaboration's tool assessing risk of bias.
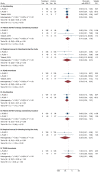
Results: The meta-analysis included 343 (lusutrombopag 3 mg, n = 173; placebo, n = 170) patients. More patients met the criteria for treatment response (platelet count at least 50 × 109/L and increase of at least 20 × 109/L from baseline anytime during the study) with lusutrombopag versus placebo (risk ratio [RR] 6.39; 95% confidence interval [CI] 3.69, 11.07; p < 0.0001). The primary efficacy outcome, proportion of patients requiring no platelet transfusion and no rescue therapy for bleeding for at least 7 days post procedure, was achieved by more patients treated with lusutrombopag versus placebo (RR 3.42; 95% CI 1.86, 6.26; p = 0.0001). The risk of any bleeding event was significantly lower with lusutrombopag compared to placebo (RR 0.55; 95% CI 0.32, 0.95; p = 0.03); conversely, thrombosis event rates were similar between lusutrombopag and placebo (RR 0.79; 95% CI 0.19, 3.24; p = 0.74).
Conclusion: This meta-analysis showed that treatment of severe thrombocytopenia with lusutrombopag in patients with CLD prior to a planned invasive procedure was efficacious and safe in increasing platelet counts, avoiding the need for platelet transfusions, and reducing risk of bleeding, thereby enhancing the certainty of evidence supporting the efficacy and safety of lusutrombopag.
Keywords: Chronic liver disease; Invasive procedure; Lusutrombopag; Meta-analysis; Severe thrombocytopenia; Thrombopoietin receptor agonist.
© 2022. The Author(s).
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

