Cholesterol-dependent cytolysins: The outstanding questions
- PMID: 35836358
- PMCID: PMC9712165
- DOI: 10.1002/iub.2661
Cholesterol-dependent cytolysins: The outstanding questions
Abstract
The cholesterol-dependent cytolysins (CDCs) are a major family of bacterial pore-forming proteins secreted as virulence factors by Gram-positive bacterial species. CDCs are produced as soluble, monomeric proteins that bind specifically to cholesterol-rich membranes, where they oligomerize into ring-shaped pores of more than 30 monomers. Understanding the details of the steps the toxin undergoes in converting from monomer to a membrane-spanning pore is a continuing challenge. In this review we summarize what we know about CDCs and highlight the remaining outstanding questions that require answers to obtain a complete picture of how these toxins kill cells.
Keywords: CD59; MACPF; cholesterol-binding protein; cholesterol-dependent cytolysin; intermedilysin; membrane-protein interactions; perfringolysin O; pneumolysin; pore-forming toxin.
© 2022 The Authors. IUBMB Life published by Wiley Periodicals LLC on behalf of International Union of Biochemistry and Molecular Biology.
Figures
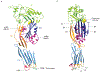
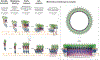
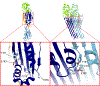
References
-
- Giddings KS, Zhao J, Sims PJ, and Tweten RK (2004) Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat. Struct. Mol. Biol 11, 1173–1178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

