Opportunities and Challenges for Alternative Nanoplasmonic Metals: Magnesium and Beyond
- PMID: 35836479
- PMCID: PMC9272400
- DOI: 10.1021/acs.jpcc.2c01944
Opportunities and Challenges for Alternative Nanoplasmonic Metals: Magnesium and Beyond
Abstract
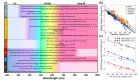
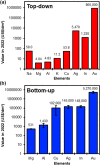
Materials that sustain localized surface plasmon resonances have a broad technology potential as attractive platforms for surface-enhanced spectroscopies, chemical and biological sensing, light-driven catalysis, hyperthermal cancer therapy, waveguides, and so on. Most plasmonic nanoparticles studied to date are composed of either Ag or Au, for which a vast array of synthetic approaches are available, leading to controllable size and shape. However, recently, alternative materials capable of generating plasmonically enhanced light-matter interactions have gained prominence, notably Cu, Al, In, and Mg. In this Perspective, we give an overview of the attributes of plasmonic nanostructures that lead to their potential use and how their performance is dictated by the choice of plasmonic material, emphasizing the similarities and differences between traditional and emerging plasmonic compositions. First, we discuss the materials limitation encapsulated by the dielectric function. Then, we evaluate how size and shape maneuver localized surface plasmon resonance (LSPR) energy and field distribution and address how this impacts applications. Next, biocompatibility, reactivity, and cost, all key differences underlying the potential of non-noble metals, are highlighted. We find that metals beyond Ag and Au are of competitive plasmonic quality. We argue that by thinking outside of the box, i.e., by looking at nonconventional materials such as Mg, one can broaden the frequency range and, more importantly, combine the plasmonic response with other properties essential for the implementation of plasmonic technologies.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Faraday M. The Bakerian Lecture: Experimental Relations of Gold (and Other Metals) to Light. Philos. Trans. R. Soc. London 1857, 147, 145–181. 10.1098/rstl.1857.0011. - DOI
-
- Thompson D. T. Michael Faraday’s Recognition of Ruby Gold: The Birth of Modern Nanotechnology. Gold Bull. 2007, 40, 267–269. 10.1007/BF03215598. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
