Modeling the Sequential Pattern Variability of the Electromotor Command System of Pulse Electric Fish
- PMID: 35836729
- PMCID: PMC9275807
- DOI: 10.3389/fninf.2022.912654
Modeling the Sequential Pattern Variability of the Electromotor Command System of Pulse Electric Fish
Abstract
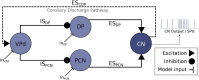
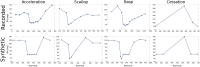
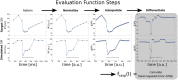
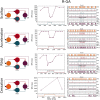
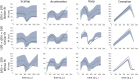
Mormyridae, a family of weakly electric fish, use electric pulses for communication and for extracting information from the environment (active electroreception). The electromotor system controls the timing of pulse generation. Ethological studies have described several sequences of pulse intervals (SPIs) related to distinct behaviors (e.g., mating or exploratory behaviors). Accelerations, scallops, rasps, and cessations are four different SPI patterns reported in these fish, each showing characteristic stereotyped temporal structures. This article presents a computational model of the electromotor command circuit that reproduces a whole set of SPI patterns while keeping the same internal network configuration. The topology of the model is based on a simplified representation of the network with four neuron clusters (nuclei). An initial configuration was built to reproduce nucleus characteristics and network topology as described by detailed morphological and electrophysiological studies. Then, a methodology based on a genetic algorithm (GA) was developed and applied to tune the model connectivity parameters to automatically reproduce a whole set of patterns recorded from freely-behaving Gnathonemus petersii specimens. Robustness analyses of input variability were performed to discard overfitting and assess validity. Results show that the set of SPI patterns is consistently reproduced reaching a dynamic balance between synaptic properties in the network. This model can be used as a tool to test novel hypotheses regarding temporal structure in electrogeneration. Beyond the electromotor model itself, the proposed methodology can be adapted to fit models of other biological networks that also exhibit sequential patterns.
Keywords: computational neuroethology; inter-pulse interval coding; multiple sequence network topology; neural sequences; temporal structure evolutionary tuning.
Copyright © 2022 Lareo, Varona and Rodriguez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

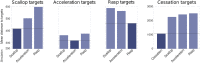
References
-
- Agapie A., Wright A. H. (2014). Theoretical analysis of steady state genetic algorithms. Appl. Math. 59, 509–525. 10.1007/s10492-014-0069-z - DOI
LinkOut - more resources
Full Text Sources

