PROPHETIC EU: Prospective Identification of Pneumonia in Hospitalized Patients in the Intensive Care Unit in European and United States Cohorts
- PMID: 35836748
- PMCID: PMC9274438
- DOI: 10.1093/ofid/ofac231
PROPHETIC EU: Prospective Identification of Pneumonia in Hospitalized Patients in the Intensive Care Unit in European and United States Cohorts
Abstract
Background: The prospective identification of patients at high risk for hospital-acquired/ventilator-associated bacterial pneumonia may improve clinical trial feasibility and foster antibacterial development. In a prior study conducted in the United States, clinical criteria were used to prospectively identify these patients; however, these criteria have not been applied in a European population.
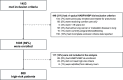
Methods: Adults considered high risk for pneumonia (treatment with ventilation or high levels of supplemental oxygen) in the intensive care units of 7 European hospitals were prospectively enrolled from June 12 to December 27, 2017. We estimated the proportion of high-risk patients developing pneumonia according to US Food and Drug Administration guidance and a subset potentially eligible for antibacterial trial enrollment. We compared patient characteristics, treatment exposures, and pneumonia incidence in a European cohort and a previously described US cohort.
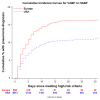
Results: Of 888 high-risk patients, 211/888 (24%) were treated for possible pneumonia, and 150/888 (17%) met the Food and Drug Administration definition for hospital-acquired/ventilator-associated bacterial pneumonia. A higher proportion of European patients treated for possible pneumonia met the pneumonia definition (150/211 [71%] vs 537/1464 [37%]; P < .001). Among patients developing pneumonia, a higher proportion of European patients met antibacterial trial eligibility criteria (124/150 [83%] vs 371/537 [69%]; P < .001).
Conclusions: Clinical criteria prospectively identified high-risk patients with high rates of pneumonia in the European cohort. Despite higher rates of established risk factors and incident pneumonia, European patients were significantly less likely to receive antibiotics for possible pneumonia than US patients. Different treatment practices may contribute to lower rates of antibacterial trial enrollment in the United States.
Keywords: antibacterial agent; bacterial pneumonia; health care–associated pneumonia; intensive care unit; mechanical ventilator.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures


References
-
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012–2017: device-associated module. Am J Infect Control 2020; 48:423–32. - PubMed
-
- Cox E, Nambiar S, Baden L. Needed: antimicrobial development. N Engl J Med 2019; 380:783–5. - PubMed
-
- Ambrose PG, Bhavnani SM, Ellis-Grosse EJ, Drusano GL. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap! Clin Infect Dis 2010; 51(Suppl 1):S103–10. - PubMed
-
- Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005; 191:2149–52. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

