Hepatotoxicity Induced by Biological Agents: Clinical Features and Current Controversies
- PMID: 35836762
- PMCID: PMC9240255
- DOI: 10.14218/JCTH.2021.00243
Hepatotoxicity Induced by Biological Agents: Clinical Features and Current Controversies
Abstract
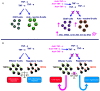
Novel biological agents including cytokines and recombinant fusion proteins are increasingly prescribed for cancer, rheumatologic, autoimmune, and inflammatory diseases, and are currently being evaluated in hepatocellular carcinoma (HCC). They are classified by their mechanism of action and include tumor necrosis factor-alpha (TNF-α) antagonists, T cell mediated antitumor inhibitors, interleukin receptor antagonists, and immune checkpoint inhibitors (ICIs). Some ICIs cause frequent hepatotoxicity with a variable clinical, biochemical, and serological presentation, especially in patients receiving another immunomodulatory agent. Half of the cases of liver damage induced by biological agents spontaneously regress after drug withdrawal, but the others require steroid therapy. Unfortunately, there are no widely accepted recommendation for the use of corticosteroids in these patients, even though international cancer societies have their own guidelines. Differentiating drug-induced autoimmune hepatitis (DIAIH) from classic AIH is challenging for pathologists, but liver biopsy is valuable, particularly in cases with unclear clinical presentation. Interesting, novel histological patterns have been described in liver damage induced by these agents (i.e., endothelitis, ring granuloma and secundary sclerosing cholangitis associated with lymphocytic infiltration of cytotoxic CD8+T cells). Here, we describe the clinical and biochemical characteristics of patients with hepatotoxicity induced by TNF-α antagonists and ICIs. Controversial issues involved in the administration of corticosteroid therapy, and hepatitis B virus (HBV) reactivation induced by immunosuppressive therapy are also discussed.
Keywords: Autoimmune hepatitis; Biologics; Checkpoint inhibitors; Drug-induced liver injury; Hepatotoxicity.
© 2022 Authors.
Conflict of interest statement
FB has been an editorial board member of Journal of Clinical and Translational Hepatology since 2019. NH has no conflict of interests related to this publication.
Figures

References
-
- Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations. Available from: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelope....
-
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548335/ - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
