Impact of National Centralized Drug Procurement Policy on Antiviral Utilization and Expenditure for Hepatitis B in China
- PMID: 35836769
- PMCID: PMC9240235
- DOI: 10.14218/JCTH.2022.00167
Impact of National Centralized Drug Procurement Policy on Antiviral Utilization and Expenditure for Hepatitis B in China
Abstract
Background and aims: The National Centralized Drug Procurement (NCDP) policy was launched in mainland China in April 2019, with entecavir (ETV) and tenofovir disoproxil fumarate (TDF) being included in the procurement list. We conducted the current study to investigate the impact of the NCDP policy on the utilization and expenditures of antiviral therapy for chronic hepatitis B (CHB) in China.
Methods: Procurement records, including monthly purchase volume, expenditure, and price of nucleos(t)ide analogs (NAs), were derived from the National Healthcare Security Administration from April 2018 to March 2021. The changes in volumes and expenditures of the first-line NAs and bid-winning products were calculated. The effects of price, volume, and structure related to drug expenditure were calculated by the Addis and Magrini (AM) Index System Analysis.
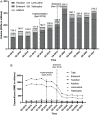
Results: The purchase volume of NAs significantly increased from 134.3 to 318.3 million DDDs, whereas the expenditure sharply decreased from 1,623.41 to 490.43 million renminbi (RMB) or 241.94 to 73.09 million US dollars (USD). The proportions of first-line NAs rose from 72.51% (ETV: 69.00%, TDF: 3.51%) to 94.97% (ETV: 77.42%, TDF: 17.55%). AM analysis showed that the NCDP policy decreased the expenditure of all NAs (S=0.91) but increased that of the first-line NAs in the bid-winning list (S=1.13). Assuming the population size of CHB patients remains stable and a compliance rate of ≥75%, the proportion of CHB patients receiving first-line antiviral therapy would increase from 6.36-8.48% to 11.56-15.41%.
Conclusions: The implementation of the NCDP policy significantly increased the utilization of first-line NAs for CHB patients at a lower expenditure. The findings provided evidence for optimizing antiviral therapy strategy and allocating medical resources in China.
Keywords: Antiviral therapy; Chronic hepatitis B; Drug expenditures; Drug utilization; National centralized drug procurement.
© 2022 Authors.
Conflict of interest statement
JJ has been an executive associate editor of Journal of Clinical and Translational Hepatology since 2021. YK has been an editorial board member of Journal of Clinical and Translational Hepatology since 2022. HY has been an editorial board member of Journal of Clinical and Translational Hepatology since 2021. The other authors have no conflict of interests related to this publication.
Figures

Similar articles
-
The Impacts of National Centralized Drug Procurement Policy on Drug Utilization and Drug Expenditures: The Case of Shenzhen, China.Int J Environ Res Public Health. 2020 Dec 15;17(24):9415. doi: 10.3390/ijerph17249415. Int J Environ Res Public Health. 2020. PMID: 33334027 Free PMC article.
-
The impact of "4 + 7" volume-based drug procurement on the volume, expenditures, and daily costs of antihypertensive drugs in Shenzhen, China: an interrupted time series analysis.BMC Health Serv Res. 2021 Nov 26;21(1):1275. doi: 10.1186/s12913-021-07143-3. BMC Health Serv Res. 2021. PMID: 34823516 Free PMC article.
-
The Effects of the National Centralized Drug Purchasing Pilot Program on Nucleos(t)ide Analogs in Shenzhen City: An Interrupted Time Series Analysis.Front Public Health. 2021 Oct 25;9:718013. doi: 10.3389/fpubh.2021.718013. eCollection 2021. Front Public Health. 2021. PMID: 34760861 Free PMC article.
-
Nucleos(t)ide Analogues for Reducing Hepatocellular Carcinoma in Chronic Hepatitis B Patients: A Systematic Review and Meta-Analysis.Gut Liver. 2020 Mar 15;14(2):232-247. doi: 10.5009/gnl18546. Gut Liver. 2020. PMID: 31158948 Free PMC article.
-
Lower risk of hepatocellular carcinoma with tenofovir than entecavir treatment in subsets of chronic hepatitis B patients: an updated meta-analysis.J Gastroenterol Hepatol. 2022 May;37(5):782-794. doi: 10.1111/jgh.15783. Epub 2022 Feb 7. J Gastroenterol Hepatol. 2022. PMID: 35080052 Review.
Cited by
-
A quasi-experimental study of the volume-based procurement (VBP) effect on antiviral medications of hepatitis B virus in China.Front Pharmacol. 2023 Sep 5;14:984794. doi: 10.3389/fphar.2023.984794. eCollection 2023. Front Pharmacol. 2023. PMID: 37731741 Free PMC article.
-
The Impact of the Definitions of Clinical Phases on the Profiles of Grey-Zone Patients with Chronic Hepatitis B Virus Infection.Viruses. 2023 May 22;15(5):1212. doi: 10.3390/v15051212. Viruses. 2023. PMID: 37243297 Free PMC article.
-
Problems and challenges encountered by Chinese medical institutions in implementing the national centralized drug procurement.Front Pharmacol. 2023 Aug 30;14:1233491. doi: 10.3389/fphar.2023.1233491. eCollection 2023. Front Pharmacol. 2023. PMID: 37745061 Free PMC article.
-
The effect of switch therapy to tenofovir versus entecavir maintenance on recurrence of hepatocellular carcinoma after surgery (SWITE): study protocol for a randomized controlled trial.Trials. 2023 Dec 2;24(1):781. doi: 10.1186/s13063-023-07822-y. Trials. 2023. PMID: 38042834 Free PMC article.
-
Comprehensive approach to controlling chronic hepatitis B in China.Clin Mol Hepatol. 2024 Apr;30(2):135-143. doi: 10.3350/cmh.2023.0412. Epub 2024 Jan 5. Clin Mol Hepatol. 2024. PMID: 38176692 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
