Advances in the clinical development of oncolytic viruses
- PMID: 35836877
- PMCID: PMC9274612
Advances in the clinical development of oncolytic viruses
Abstract
Objectives: Oncolytic viruses (OVs) are natural or recombinant viruses that selectively infect and kill cancer cells without harming normal cells. This review aimed to explore some ongoing and completed clinical studies on OVs, in China and worldwide, to depict a comprehensive landscape of OV clinical trials, and summarize the existing evidence on safety and effectiveness of oncolytic therapy against tumors.
Methods: Used the Center for Drug Evaluation of China National Medical Products Administration (NMPA), Chinese Clinical Trial Registry (ChiCTR), International Clinical Trials Registry Platform, ClinicalTrials.gov website, China National Knowledge Infrastructure (CNKI), and PubMed.
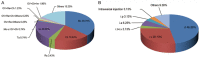
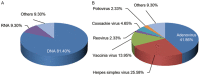
Results: As of October 1, 2021, 408 clinical trials on 31 OV products have been conducted, with oncolytic DNA viruses being the most investigated ones; phase I and phase II clinical studies accounted for approximately 80% of all studies. Published clinical studies have shown that OVs, such as H101, T-VEC, G47Δ, OH2, T3011, and Pelareorep, have significant anti-tumor effects on various tumors, with only mild adverse events. When OVs are used together with antiviral drugs in the clinic, drug interactions should be considered based on the sensitivity of OVs to antiviral drugs.
Conclusions: OVs exhibit accurate oncolysis and favorable safety, and have positive effects on a variety of tumor treatments. It is worth noting that most of the OVs under development are still in their early stages, which is both a challenge and a promising prospect.
Keywords: Oncolytic viruses; antiviral drugs; immunotherapy; tumor; virotherapy.
AJTR Copyright © 2022.
Conflict of interest statement
None.
Figures

References
-
- Gujar S, Bell J, Diallo JS. SnapShot: cancer immunotherapy with oncolytic viruses. Cell. 2019;176:1240–1240. e1. - PubMed
-
- Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192:415. - PubMed
-
- Goradel NH, Baker AT, Arashkia A, Ebrahimi N, Ghorghanlu S, Negahdari B. Oncolytic virotherapy: challenges and solutions. Curr Probl Cancer. 2021;45:100639. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
