Photoactivation of a Mechanosensitive Channel
- PMID: 35836929
- PMCID: PMC9273776
- DOI: 10.3389/fmolb.2022.905306
Photoactivation of a Mechanosensitive Channel
Abstract
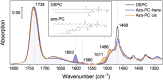
Optogenetics in the conventional sense, i.e. the use of engineered proteins that gain their light sensitivity from naturally abundant chromophores, represents an exciting means to trigger and control biological activity by light. As an alternate approach, photopharmacology controls biological activity with the help of synthetic photoswitches. Here, we used an azobenzene-derived lipid analogue to optically activate the transmembrane mechanosensitive channel MscL which responds to changes in the lateral pressure of the lipid bilayer. In this work, MscL has been reconstituted in nanodiscs, which provide a native-like environment to the protein and a physical constraint to membrane expansion. We characterized this photomechanical system by FTIR spectroscopy and assigned the vibrational bands of the light-induced FTIR difference spectra of the trans and cis states of the azobenzene photolipid by DFT calculations. Differences in the amide I range indicated reversible conformational changes in MscL as a direct consequence of light switching. With the mediation of nanodiscs, we inserted the transmembrane protein in a free standing photoswitchable lipid bilayer, where electrophysiological recordings confirmed that the ion channel could be set to one of its sub-conducting states upon light illumination. In conclusion, a novel approach is presented to photoactivate and control cellular processes as complex and intricate as gravitropism and turgor sensing in plants, contractility of the heart, as well as sensing pain, hearing, and touch in animals.
Keywords: AzoPC; FTIR spectroscopy; Langmuir film; MscL; biomembrane; electrophysiology; nanodiscs; photolipids.
Copyright © 2022 Crea, Vorkas, Redlich, Cruz, Shi, Trauner, Lange, Schlesinger and Heberle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bayburt T. H., Grinkova Y. V., Sligar S. G. (2002). Self-assembly of Discoidal Phospholipid Bilayer Nanoparticles with Membrane Scaffold Proteins. Nano Lett. 2, 853–856. 10.1021/nl025623k - DOI
LinkOut - more resources
Full Text Sources
Research Materials

