Wnt3a knockdown promotes collagen type II expression in rat chondrocytes
- PMID: 35837029
- PMCID: PMC9257960
- DOI: 10.3892/etm.2022.11453
Wnt3a knockdown promotes collagen type II expression in rat chondrocytes
Abstract
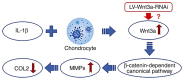
Osteoarthritis (OA) is a chronic condition caused by cartilage degradation, and there are currently no effective methods for preventing the progression of this disease; gene therapy is a relatively novel method for treating arthritis. Decreased collagen type II (Col2) expression within the cartilage matrix is an important factor for the development of OA, and Wnt3a serves a significant role in cartilage homeostasis. The present study assessed whether Wnt3a knockdown promoted Col2 expression in chondrocytes. Lentivirus-introduced small interfering RNA was used to knock down the expression of Wnt3a in primary rat chondrocytes, and then IL-1β treatment was used to establish an OA chondrocyte model. The expression of target genes (Wnt3a, Col2, MMP-13 and β-catenin) was analyzed using reverse transcription-quantitative PCR, western blotting and immunocytochemistry. There was significantly less MMP-13 and β-catenin expression in the Wnt3a knockdown cells compared with the other controls. Col2 expression was significantly higher in the Wnt3a-knockdown cells compared with the control cells, indicating that knockdown of Wnt3a may promote Col2 expression. Consequently, Wnt3a was indicated to be an important factor in cartilage homeostasis, and Wnt3a knockdown may serve as a novel method for OA therapy.
Keywords: Wnt3a; chondrocyte; collagen type II; knockdown.
Copyright: © Shi et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
BCP crystals promote chondrocyte hypertrophic differentiation in OA cartilage by sequestering Wnt3a.Ann Rheum Dis. 2020 Jul;79(7):975-984. doi: 10.1136/annrheumdis-2019-216648. Epub 2020 May 5. Ann Rheum Dis. 2020. PMID: 32371389
-
Silencing of Wnt5a prevents interleukin-1β-induced collagen type II degradation in rat chondrocytes.Exp Ther Med. 2016 Nov;12(5):3161-3166. doi: 10.3892/etm.2016.3788. Epub 2016 Oct 11. Exp Ther Med. 2016. PMID: 27882132 Free PMC article.
-
MicroRNA-497-5p attenuates IL-1β-induced cartilage matrix degradation in chondrocytes via Wnt/β-catenin signal pathway.Int J Clin Exp Pathol. 2019 Aug 1;12(8):3108-3118. eCollection 2019. Int J Clin Exp Pathol. 2019. PMID: 31934153 Free PMC article.
-
Xanthan Gum Ameliorates Osteoarthritis and Mitigates Cartilage Degradation via Regulation of the Wnt3a/β-Catenin Signaling Pathway.Med Sci Monit. 2019 Oct 6;25:7488-7498. doi: 10.12659/MSM.916092. Med Sci Monit. 2019. PMID: 31587011 Free PMC article.
-
Effects of Wnt3A and mechanical load on cartilage chondrocyte homeostasis.Arthritis Res Ther. 2011;13(6):R203. doi: 10.1186/ar3536. Epub 2011 Dec 9. Arthritis Res Ther. 2011. PMID: 22151902 Free PMC article.
References
-
- Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, Hoy D, Ashrafi-Asgarabad A, Sepidarkish M, Almasi-Hashiani A, et al. Global, regional and national burden of osteoarthritis 1990-2017: A systematic analysis of the global burden of disease study 2017. Ann Rheum Dis. 2020;79:819–828. doi: 10.1136/annrheumdis-2019-216515. - DOI - PubMed
-
- Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, Imoto AM, Toupin-April K, Westby M, Gallardo ICÁ, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part one: Introduction, and mind-body exercise programs. Clin Rehabil. 2017;31:582–595. doi: 10.1177/0269215517691083. - DOI - PubMed
-
- Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D, et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72:220–233. doi: 10.1002/acr.24131. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
