Metformin mitigates gas explosion-induced blast lung injuries through AMPK-mediated energy metabolism and NOX2-related oxidation pathway in rats
- PMID: 35837050
- PMCID: PMC9257965
- DOI: 10.3892/etm.2022.11456
Metformin mitigates gas explosion-induced blast lung injuries through AMPK-mediated energy metabolism and NOX2-related oxidation pathway in rats
Abstract
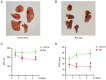
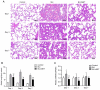
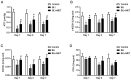
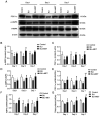
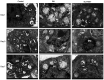
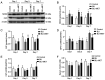
Gas explosions are a recurrent event in coal mining that cause severe pulmonary damage due to shock waves, and there is currently no effective targeted treatment. To illustrate the mechanism of gas explosion-induced lung injury and to explore strategies for blast lung injury (BLI) treatment, the present study used a BLI rat model and supplementation with metformin (MET), an AMP-activated protein kinase (AMPK) activator, at a dose of 10 mg/kg body weight by intraperitoneal injection. Protein expression levels were detected by western blotting. Significantly decreased expression of phosphorylated (p)-AMPK, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α) and metabolic activity were observed in the BLI group compared with those in the control group. However, the mitochondrial stability, metabolic activity and expression of p-AMPK and PGC1α were elevated following MET treatment. These results suggested that MET could attenuate gas explosion-induced BLI by improving mitochondrial homeostasis. Meanwhile, high expression of nicotinamide adenine dinucleotide phosphate oxidase (NOX2) and low expression of catalase (CAT) were observed in the BLI group. The expression levels of NOX2 and CAT were restored in the BLI + MET group relative to changes in the BLI group, and the accumulation of oxidative stress was successfully reversed following MET treatment. Overall, these findings revealed that MET could alleviate BLI by activating the AMPK/PGC1α pathway and inhibiting oxidative stress caused by NOX2 activation.
Keywords: AMP-activated protein kinase; blast lung injury; gas explosion injury; metformin; mitochondria; nicotinamide adenine dinucleotide phosphate oxidase.
Copyright: © Zhang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway.Adipocyte. 2020 Dec;9(1):484-494. doi: 10.1080/21623945.2020.1807850. Adipocyte. 2020. PMID: 32835596 Free PMC article.
-
Fucoxanthin Protects against oxLDL-Induced Endothelial Damage via Activating the AMPK-Akt-CREB-PGC1α Pathway.Mol Nutr Food Res. 2019 May;63(10):e1801353. doi: 10.1002/mnfr.201801353. Epub 2019 Apr 10. Mol Nutr Food Res. 2019. PMID: 30892786
-
Activation of AMPK attenuates LPS-induced acute lung injury by upregulation of PGC1α and SOD1.Exp Ther Med. 2016 Sep;12(3):1551-1555. doi: 10.3892/etm.2016.3465. Epub 2016 Jun 17. Exp Ther Med. 2016. PMID: 27602077 Free PMC article.
-
Sensing and responding to energetic stress: The role of the AMPK-PGC1α-NRF1 axis in control of mitochondrial biogenesis in fish.Comp Biochem Physiol B Biochem Mol Biol. 2016 Sep;199:4-12. doi: 10.1016/j.cbpb.2015.09.005. Epub 2015 Sep 21. Comp Biochem Physiol B Biochem Mol Biol. 2016. PMID: 26393435
-
An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy.Int J Cardiol. 2013 Oct 9;168(4):3160-72. doi: 10.1016/j.ijcard.2013.07.150. Epub 2013 Aug 6. Int J Cardiol. 2013. PMID: 23932046 Review.
Cited by
-
Effects of metformin on acute respiratory distress syndrome in preclinical studies: a systematic review and meta-analysis.Front Pharmacol. 2023 Sep 28;14:1215307. doi: 10.3389/fphar.2023.1215307. eCollection 2023. Front Pharmacol. 2023. PMID: 37841910 Free PMC article.
-
Fecal microbiota transplantation alleviates cognitive impairment by improving gut microbiome composition and barrier function in male rats of traumatic brain injury following gas explosion.Front Microbiol. 2024 Nov 1;15:1485936. doi: 10.3389/fmicb.2024.1485936. eCollection 2024. Front Microbiol. 2024. PMID: 39552646 Free PMC article.
-
Assessment model of blast injury: A narrative review.iScience. 2025 Jun 6;28(7):112830. doi: 10.1016/j.isci.2025.112830. eCollection 2025 Jul 18. iScience. 2025. PMID: 40612508 Free PMC article. Review.
References
-
- Zhang Z, Li H, Liang Z, Li C, Yang Z, Li Y, Cao L, She Y, Wang W, Liu C, Chen L. Vaporized perfluorocarbon inhalation attenuates primary blast lung injury in canines by inhibiting mitogen-activated protein kinase/nuclear factor-κB activation and inducing nuclear factor, erythroid 2 like 2 pathway. Toxicol Lett. 2020;319:49–57. doi: 10.1016/j.toxlet.2019.10.019. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
