LncRNA NEAT1/microRNA-124 regulates cell viability, inflammation and fibrosis in high-glucose-treated mesangial cells
- PMID: 35837070
- PMCID: PMC9257954
- DOI: 10.3892/etm.2022.11434
LncRNA NEAT1/microRNA-124 regulates cell viability, inflammation and fibrosis in high-glucose-treated mesangial cells
Abstract
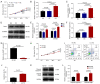
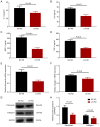
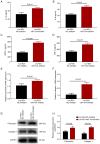
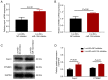
Long non-coding RNA (lncRNA) nuclear enriched abundant transcript 1 (NEAT1) has been frequently found to be dysregulated, which contributes to diabetes-related complications. The present study aimed to explore the effect of knockdown on mouse mesangial cell (MMC) viability, apoptosis, inflammation and fibrosis in an in vitro model of diabetic nephropathy (DN). The SV40 MES13 MMC cell line was first cultured with high glucose to establish an in vitro MMC DN cell model. Lnc-NEAT1 shRNA or the negative control shRNA were transfected into MMC DN cells, followed by the measurement of cell viability, apoptosis, inflammation, fibrosis and microRNA (miR)-124 expression, a known target of lnc-NEAT1, using Cell Counting Kit-8, flow cytometry, ELISA, western blotting [Capain1 (capn1), β-catenin (CTNNB1), cleaved caspase 3, cleaved poly-(ADP ribose) polymerase, fibronectin and Collagen] and reverse transcription-quantitative PCR (Capn1, CTNNB1, lnc-NEAT1, fibronectin, collagen and miR-124), respectively. In rescue experiments, the miR-124 and negative control inhibitor were co-transfected into lnc-NEAT1-downregulated cells, following which cell viability, apoptosis, inflammation, fibrosis, capn1 and CTNNB1 expression were measured. Lnc-NEAT1 expression was increased in high glucose-treated cells compared with that in normal glucose-treated cells and osmotic control cells, suggesting that lnc-NEAT1 is overexpressed in the MMC DN cell model. In the MMC DN cell model, lncRNA-NEAT1 knockdown enhanced cell apoptosis but reduced cell viability and the secretion of inflammatory cytokines in the supernatant (IL-1β, IL-8, monocyte chemotactic protein 1 and TNF-α), in addition to reducing the expression of fibrosis markers fibronectin and collagen I in the lysates. Lnc-NEAT1 knockdown increased miR-124 expression. Furthermore, transfection with the miR-124 inhibitor reduced cell apoptosis but increased cell viability, inflammation and fibrosis in lnc-NEAT1-downregulated MMC DN cells. miR-124 inhibitor transfection also increased the expression levels of Capn1 and CTNNB1. Taken together, the findings of the present study demonstrated that lnc-NEAT1 knockdown was able to attenuate MMC viability, inflammation and fibrosis by regulating miR-124 expression and the Capn1/β-catenin signaling pathway downstream. Therefore, Lnc-NEAT1 may serve as a potential therapeutic target for DN.
Keywords: calpain 1; diabetic nephropathy; lncRNA nuclear enriched abundant transcript 1; mesangial cell; microRNA-124; β-catenin.
Copyright: © Zhao et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Downregulation of lncRNA NEAT1 inhibits mouse mesangial cell proliferation, fibrosis, and inflammation but promotes apoptosis in diabetic nephropathy.Int J Clin Exp Pathol. 2019 Apr 1;12(4):1174-1183. eCollection 2019. Int J Clin Exp Pathol. 2019. PMID: 31933932 Free PMC article.
-
Knock-Down of Long Non-Coding RNA ANRIL Suppresses Mouse Mesangial Cell Proliferation, Fibrosis, Inflammation via Regulating Wnt/β-Catenin and MEK/ERK Pathways in Diabetic Nephropathy.Exp Clin Endocrinol Diabetes. 2022 Jan;130(1):30-36. doi: 10.1055/a-1185-9283. Epub 2020 Jul 29. Exp Clin Endocrinol Diabetes. 2022. PMID: 32726814
-
LncRNA NEAT1 Accelerates the Proliferation, Oxidative Stress, Inflammation, and Fibrosis and Suppresses the Apoptosis Through the miR-423-5p/GLIPR2 Axis in Diabetic Nephropathy.J Cardiovasc Pharmacol. 2022 Mar 1;79(3):342-354. doi: 10.1097/FJC.0000000000001177. J Cardiovasc Pharmacol. 2022. PMID: 34803150
-
Defining the transcriptional routes controlling lncRNA NEAT1 expression: implications in cellular stress response, inflammation, and differentiation.Discov Oncol. 2025 May 15;16(1):768. doi: 10.1007/s12672-025-02510-6. Discov Oncol. 2025. PMID: 40369379 Free PMC article. Review.
-
Mechanism of action of lncRNA-NEAT1 in immune diseases.Front Genet. 2025 Mar 5;16:1501115. doi: 10.3389/fgene.2025.1501115. eCollection 2025. Front Genet. 2025. PMID: 40110044 Free PMC article. Review.
Cited by
-
Mechanism of action of non-coding RNAs and traditional Chinese medicine in myocardial fibrosis: Focus on the TGF-β/Smad signaling pathway.Front Pharmacol. 2023 Feb 9;14:1092148. doi: 10.3389/fphar.2023.1092148. eCollection 2023. Front Pharmacol. 2023. PMID: 36843918 Free PMC article. Review.
-
Research progress on miR-124-3p in the field of kidney disease.BMC Nephrol. 2024 Aug 7;25(1):252. doi: 10.1186/s12882-024-03688-7. BMC Nephrol. 2024. PMID: 39112935 Free PMC article. Review.
-
MicroRNA‑203a‑3p improves bleomycin and pingyangmycin sensitivity by inactivating the PI3K/AKT pathway in hemangioma.Exp Ther Med. 2024 Jan 4;27(2):80. doi: 10.3892/etm.2024.12369. eCollection 2024 Feb. Exp Ther Med. 2024. PMID: 38274341 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
