Comparative Analysis of Single-Cell RNA Sequencing Platforms and Methods
- PMID: 35837267
- PMCID: PMC9258609
- DOI: 10.7171/3fc1f5fe.3eccea01
Comparative Analysis of Single-Cell RNA Sequencing Platforms and Methods
Abstract
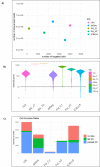
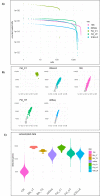
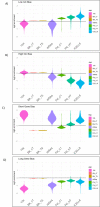
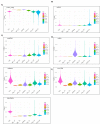
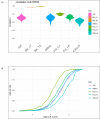
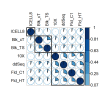
Single-cell RNA sequencing (scRNA-seq) offers great new opportunities for increasing our understanding of complex biological processes. In particular, development of an accurate Human Cell Atlas is largely dependent on the rapidly advancing technologies and molecular chemistries employed in scRNA-seq. These advances have already allowed an increase in throughput for scRNA-seq from 96 to 80,000 cells on a single instrument run by capturing cells within nanoliter droplets. Although this increase in throughput is critical for many experimental questions, a thorough comparison between microfluidic-based, plate-based, and droplet-based technologies or between multiple available platforms utilizing these technologies is largely lacking. Here, we report scRNA-seq data from SUM149PT cells treated with the histone deacetylase inhibitor trichostatin A versus untreated controls across several scRNA-seq platforms (Fluidigm C1, WaferGen iCell8, 10x Genomics Chromium Controller, and Illumina/BioRad ddSEQ). The primary goal of this project was to demonstrate RNA sequencing methods for profiling the ultra-low amounts of RNA present in individual cells, and this report discusses the results of the study, as well as technical challenges and lessons learned and present general guidelines for best practices in sample preparation and analysis.
Keywords: RNA-seq; platforms; single cell.
Copyright ©️ 2021 Association of Biomolecular ResourceFacilities. All rights reserved.
Conflict of interest statement
Conflict of Interest Disclosures: The authors declare no conflicts of interest.
Figures


References
-
- Sen R, Dolgalev I, Bayin NS, Heguy A, Tsirigos A, Placantonakis DG. Single-cell RNA sequencing of glioblastoma cells. Methods Mol Biol 2018;1741:151–170. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
