Central Gαi2 Protein Mediated Neuro-Hormonal Control of Blood Pressure and Salt Sensitivity
- PMID: 35837296
- PMCID: PMC9275552
- DOI: 10.3389/fendo.2022.895466
Central Gαi2 Protein Mediated Neuro-Hormonal Control of Blood Pressure and Salt Sensitivity
Abstract
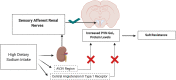
Hypertension, a major public health issue, is estimated to contribute to 10% of all deaths worldwide. Further, the salt sensitivity of blood pressure is a critical risk factor for the development of hypertension. The hypothalamic paraventricular nucleus (PVN) coordinates neuro-hormonal responses to alterations in plasma sodium and osmolality and multiple G Protein-Coupled Receptors (GPCRs) are involved in fluid and electrolyte homeostasis. In acute animal studies, our laboratory has shown that central Gαi/o subunit protein signal transduction mediates hypotensive and bradycardic responses and that Gz/q, proteins mediate the release of arginine vasopressin (AVP) and subsequent aquaretic responses to acute pharmacological stimuli. Extending these studies, our laboratory has shown that central Gαi2 proteins selectively mediate the hypotensive, sympathoinhibitory and natriuretic responses to acute pharmacological activation of GPCRs and in response to acute physiological challenges to fluid and electrolyte balance. In addition, following chronically elevated dietary sodium intake, salt resistant rats demonstrate site-specific and subunit-specific upregulation of Gαi2 proteins in the PVN, resulting in sympathoinhibition and normotension. In contrast, chronic dietary sodium intake in salt sensitive animals, which fail to upregulate PVN Gαi2 proteins, results in the absence of dietary sodium-evoked sympathoinhibition and salt sensitive hypertension. Using in situ hybridization, we observed that Gαi2 expressing neurons in parvocellular division of the PVN strongly (85%) colocalize with GABAergic neurons. Our data suggest that central Gαi2 protein-dependent responses to an acute isotonic volume expansion (VE) and elevated dietary sodium intake are mediated by the peripheral sensory afferent renal nerves and do not depend on the anteroventral third ventricle (AV3V) sodium sensitive region or the actions of central angiotensin II type 1 receptors. Our translational human genomic studies have identified three G protein subunit alpha I2 (GNAI2) single nucleotide polymorphisms (SNPs) as potential biomarkers in individuals with salt sensitivity and essential hypertension. Collectively, PVN Gαi2 proteins-gated pathways appear to be highly conserved in salt resistance to counter the effects of acute and chronic challenges to fluid and electrolyte homeostasis on blood pressure via a renal sympathetic nerve-dependent mechanism.
Keywords: Gαi2 proteins; hypertension; paraventricular nucleus; renal nerves; salt sensitivity.
Copyright © 2022 Amraei, Moreira and Wainford.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Sensory Afferent Renal Nerve Activated Gαi2 Subunit Proteins Mediate the Natriuretic, Sympathoinhibitory and Normotensive Responses to Peripheral Sodium Challenges.Front Physiol. 2021 Nov 30;12:771167. doi: 10.3389/fphys.2021.771167. eCollection 2021. Front Physiol. 2021. PMID: 34916958 Free PMC article.
-
Hypothalamic Paraventricular Nucleus Gαi2 (Guanine Nucleotide-Binding Protein Alpha Inhibiting Activity Polypeptide 2) Protein-Mediated Neural Control of the Kidney and the Salt Sensitivity of Blood Pressure.Hypertension. 2020 Apr;75(4):1002-1011. doi: 10.1161/HYPERTENSIONAHA.119.13777. Epub 2020 Mar 9. Hypertension. 2020. PMID: 32148128 Free PMC article.
-
Neuroanatomical characterization of Gαi2-expressing neurons in the hypothalamic paraventricular nucleus of male and female Sprague-Dawley rats.Physiol Genomics. 2021 Jan 1;53(1):12-21. doi: 10.1152/physiolgenomics.00097.2020. Epub 2020 Nov 30. Physiol Genomics. 2021. PMID: 33252993 Free PMC article.
-
Brain Gαi 2 -subunit proteins and the prevention of salt sensitive hypertension.Front Physiol. 2015 Aug 19;6:233. doi: 10.3389/fphys.2015.00233. eCollection 2015. Front Physiol. 2015. PMID: 26347659 Free PMC article. Review.
-
Angiotensin and osmoreceptor inputs to the area postrema: role in long-term control of fluid homeostasis and arterial pressure.Clin Exp Pharmacol Physiol. 2000 May-Jun;27(5-6):443-9. doi: 10.1046/j.1440-1681.2000.03263.x. Clin Exp Pharmacol Physiol. 2000. PMID: 10831251 Review.
Cited by
-
An Overview on Renal and Central Regulation of Blood Pressure by Neuropeptide FF and Its Receptors.Int J Mol Sci. 2024 Dec 11;25(24):13284. doi: 10.3390/ijms252413284. Int J Mol Sci. 2024. PMID: 39769048 Free PMC article. Review.
-
Central nervous system mechanisms of salt-sensitive hypertension.Physiol Rev. 2025 Oct 1;105(4):1989-2032. doi: 10.1152/physrev.00035.2024. Epub 2025 May 2. Physiol Rev. 2025. PMID: 40315132 Review.
References
-
- Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C, et al. . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (2018) 71(6):1269–324. doi: 10.1161/HYP.0000000000000066 - DOI - PubMed
-
- Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. . Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association's Strategic Impact Goal Through 2020 and Beyond. Circulation (2010) 121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

