Manufacturing artificial bone allografts: a perspective
- PMID: 35837344
- PMCID: PMC9255790
- DOI: 10.12336/biomatertransl.2022.01.007
Manufacturing artificial bone allografts: a perspective
Abstract
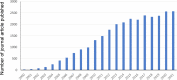
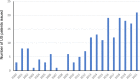
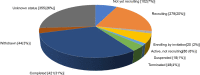
Bone grafts have traditionally come from four sources: the patients' own tissue (autograft), tissue from a living or cadaveric human donor (allograft), animal donors (xenograft) and synthetic artificial biomaterials (ceramics, cement, polymers, and metal). However, all of these have advantages and drawbacks. The most commercially successful bone grafts so far are allografts, which hold 57% of the current bone graft market; however, disease transmission and scarcity are still significant drawbacks limiting their use. Tissue-engineered grafts have great potential, in which human stem cells and synthetical biomaterials are combined to produce bone-like tissue in vitro, but this is yet to be approved for widespread clinical practice. It is hypothesised that artificial bone allografts can be mass-manufactured to replace conventional bone allografts through refined bone tissue engineering prior to decellularisation. This review article aims to review current literature on (1) conventional bone allograft preparation; (2) bone tissue engineering including the use of synthetic biomaterials as bone graft substitute scaffolds, combined with osteogenic stem cells in vitro; (3) potential artificial allograft manufacturing processes, including mass production of engineered bone tissue, osteogenic enhancement, decellularisation, sterilisation and safety assurance for regulatory approval. From these assessments, a practical route map for mass production of artificial allografts for clinical use is proposed.
Keywords: biomaterials; bone graft; decellularisation; stem cells; tissue engineering.
Conflict of interest statement
Conflicts of interest statement: The authors declare no competing interests. Editor note: Zhidao Xia is an Editorial Board members of Biomaterials Translational. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer review handled independently of this Editorial Board member and his research group.
Figures


References
-
- Brydone A. S., Meek D., Maclaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010;224:1329–1343. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
