Plasmablast Expansion Following the Tetravalent, Live-Attenuated Dengue Vaccine Butantan-DV in DENV-Naïve and DENV-Exposed Individuals in a Brazilian Cohort
- PMID: 35837409
- PMCID: PMC9274664
- DOI: 10.3389/fimmu.2022.908398
Plasmablast Expansion Following the Tetravalent, Live-Attenuated Dengue Vaccine Butantan-DV in DENV-Naïve and DENV-Exposed Individuals in a Brazilian Cohort
Abstract
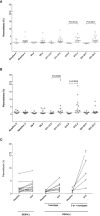
An effective vaccine against the dengue virus (DENV) should induce a balanced, long-lasting antibody (Ab) response against all four viral serotypes. The burst of plasmablasts in the peripheral blood after vaccination may reflect enriched vaccine-specific Ab secreting cells. Here we characterize the acute plasmablast responses from naïve and DENV-exposed individuals following immunization with the live attenuated tetravalent (LAT) Butantan DENV vaccine (Butantan-DV). The frequency of circulating plasmablasts was determined by flow cytometric analysis of fresh whole blood specimens collected from 40 participants enrolled in the Phase II Butantan-DV clinical trial (NCT01696422) before and after (days 6, 12, 15 and 22) vaccination. We observed a peak in the number of circulating plasmablast at day 15 after vaccination in both the DENV naïve and the DENV-exposed vaccinees. DENV-exposed vaccinees experienced a significantly higher plasmablast expansion. In the DENV-naïve vaccinees, plasmablasts persisted for approximately three weeks longer than among DENV-exposed volunteers. Our findings indicate that the Butantan-DV can induce plasmablast responses in both DENV-naïve and DENV-exposed individuals and demonstrate the influence of pre-existing DENV immunity on Butantan DV-induced B-cell responses.
Keywords: dengue; dengue infection; dengue vaccine; humoral response; plasmablast.
Copyright © 2022 Silveira, Magnani, Costa, Avelino-Silva, Ricciardi, Timenetsky, Goulart, Correia, Marmorato, Ferrari, Nakagawa, Tomiyama, Tomiyama, Kalil, Palacios, Precioso, Watkins and Kallás.
Conflict of interest statement
JK and RP are former employees of the Butantan Institute. AP is an employee of the Butantan Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer (CP) declared a shared affiliation with the author (MT) to the handling editor at the time of review.
Figures



References
-
- (2020). Available at: https://www.saude.gov.br/images/pdf/2020/August/21/Boletim-epidemiologic....
-
- (2021), 1–17 p. Secretaria de Vigilância em Saúde, Saúde M da. Monitoramento dos casos de arboviroses pelo mosquito Aedes (dengue, chikungunya urbanas causados por vírus transmitidos e zika), semanas epidemiológicas 1 a 51, 2021.
-
- (2020), 1–13 p. Saúde S de V em, Saúde M da. Boletim epidemiológico. Monitoramento dos casos de Arboviroses urbanas transmitidas pelo Aedes (dengue, chikungunya e Zika), semanas epidemiológicas 1 a 50, 2020.
-
- (2022) 2022. Secretaria de Vigilância em Saúde M da S. Monitoramento dos casos de arboviroses até a semana epidemiológica 9 de.
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

