Gene Therapy in Orthopaedics: Progress and Challenges in Pre-Clinical Development and Translation
- PMID: 35837555
- PMCID: PMC9274665
- DOI: 10.3389/fbioe.2022.901317
Gene Therapy in Orthopaedics: Progress and Challenges in Pre-Clinical Development and Translation
Abstract
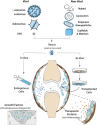
In orthopaedics, gene-based treatment approaches are being investigated for an array of common -yet medically challenging- pathologic conditions of the skeletal connective tissues and structures (bone, cartilage, ligament, tendon, joints, intervertebral discs etc.). As the skeletal system protects the vital organs and provides weight-bearing structural support, the various tissues are principally composed of dense extracellular matrix (ECM), often with minimal cellularity and vasculature. Due to their functional roles, composition, and distribution throughout the body the skeletal tissues are prone to traumatic injury, and/or structural failure from chronic inflammation and matrix degradation. Due to a mixture of environment and endogenous factors repair processes are often slow and fail to restore the native quality of the ECM and its function. In other cases, large-scale lesions from severe trauma or tumor surgery, exceed the body's healing and regenerative capacity. Although a wide range of exogenous gene products (proteins and RNAs) have the potential to enhance tissue repair/regeneration and inhibit degenerative disease their clinical use is hindered by the absence of practical methods for safe, effective delivery. Cumulatively, a large body of evidence demonstrates the capacity to transfer coding sequences for biologic agents to cells in the skeletal tissues to achieve prolonged delivery at functional levels to augment local repair or inhibit pathologic processes. With an eye toward clinical translation, we discuss the research progress in the primary injury and disease targets in orthopaedic gene therapy. Technical considerations important to the exploration and pre-clinical development are presented, with an emphasis on vector technologies and delivery strategies whose capacity to generate and sustain functional transgene expression in vivo is well-established.
Keywords: MSC mesenchymal stromal cell; gene therapy; gene transfer; orthopaedics; regenerative medicine; viral vector.
Copyright © 2022 Watson-Levings, Palmer, Levings, Dacanay, Evans and Ghivizzani.
Conflict of interest statement
SG and CE are founders, shareholders, and scientific advisory board members of Genascence Inc., a company developing gene-based treatments for osteoarthritis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Aghaloo T., Jiang X., Soo C., Zhang Z., Zhang X., Hu J., et al. (2007). A Study of the Role of Nell-1 Gene Modified Goat Bone Marrow Stromal Cells in Promoting New Bone Formation. Mol. Ther. 15, 1872–1880. 10.1038/sj.mt.6300270 PubMed Abstract | 10.1038/sj.mt.6300270 | Google Scholar - DOI - PMC - PubMed
-
- Alaee F., Bartholomae C., Sugiyama O., Virk M., Drissi H., Wu Q., et al. (2015). Biodistribution of LV-TSTA Transduced Rat Bone Marrow Cells Used for "Ex-Vivo" Regional Gene Therapy for Bone Repair. Cgt 15, 481–491. 10.2174/1566523215666150812120229 10.2174/1566523215666150812120229 | Google Scholar - DOI - PubMed
-
- Alba R., Bosch A., Chillon M. (2005). Gutless Adenovirus: Last-Generation Adenovirus for Gene Therapy. Gene Ther. 12 (Suppl. 1), S18–S27. 10.1038/sj.gt.3302612 PubMed Abstract | 10.1038/sj.gt.3302612 | Google Scholar - DOI - PubMed
-
- Alluri R., Song X., Bougioukli S., Pannell W., Vakhshori V., Sugiyama O., et al. (2019). Regional Gene Therapy with 3D Printed Scaffolds to Heal Critical Sized Bone Defects in a Rat Model. J. Biomed. Mater Res. 107, 2174–2182. 10.1002/jbm.a.36727 10.1002/jbm.a.36727 | Google Scholar - DOI - PMC - PubMed
-
- Altawalah H. (2021). Antibody Responses to Natural SARS-CoV-2 Infection or after COVID-19 Vaccination. Vaccines (Basel) 9, 910. 10.3390/vaccines9080910 PubMed Abstract | 10.3390/vaccines9080910 | Google Scholar - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

