A Profile of Avelumab Plus Axitinib in the Treatment of Renal Cell Carcinoma
- PMID: 35837579
- PMCID: PMC9275425
- DOI: 10.2147/TCRM.S263832
A Profile of Avelumab Plus Axitinib in the Treatment of Renal Cell Carcinoma
Abstract
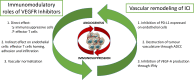
Until recently, the approved first-line treatment for metastatic RCC (mRCC) consisted of tyrosine kinase inhibitors (TKI) targeting the vascular endothelial growth factor receptors (VEGFR) monotherapy. The landscape of first-line treatment has been transformed in the last few years with the advent of immune checkpoint inhibitors (ICI) or VEGFR TKI plus ICI combinations. This article focuses on the profile of one of these ICI plus VEGFR TKI combination, avelumab plus axitinib. We detail the characteristics of each drug separately, and then we explore the rationale for their association, its efficacy and the resulting toxicity. Finally, we examine the factors associated with avelumab plus axitinib outcomes, and their impact on therapeutic strategy.
Keywords: avelumab; axitinib; immune checkpoint inhibitor; pharmacology; renal cell carcinoma; vascular endothelial growth factor.
© 2022 Tiako Meyo et al.
Conflict of interest statement
Pr. Goldwasser reports grants and personal fees from BAXTER, and personal fees from FRESENIUS. KABI, and NUTRICIA, outside the submitted work. Dr. Huillard reports personal fees from BAYER, SANOFI, MSD, BMS, IPSEN, PFIZER, EISAI, JANSSEN, and ASTRA ZENECA, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources