Plasma Exosomal S1PR5 and CARNS1 as Potential Non-invasive Screening Biomarkers of Coronary Heart Disease
- PMID: 35837598
- PMCID: PMC9273894
- DOI: 10.3389/fcvm.2022.845673
Plasma Exosomal S1PR5 and CARNS1 as Potential Non-invasive Screening Biomarkers of Coronary Heart Disease
Abstract
Background: Early diagnosis and treatment significantly improve the prognosis of coronary heart disease (CHD), but no convenient screening tools are available. This study aims to find potential non-invasive screening biomarkers of coronary heart disease.
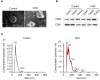
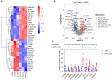
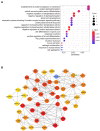
Method: We performed microarray analysis to investigate the mRNA expression levels in Small extracellular vesicles (sEVs) and screen significantly differentially expressed mRNAs in CHD patients vs. non-CHD patients. We then performed quantitative real-time polymerase chain reaction (qRT-PCR) to validate the microarray results, and we calculated the correlations between expression levels and clinicopathological data. Microarray analysis identified 72 downregulated mRNAs and 31 upregulated mRNAs in CHD patients relative to non-CHD patients.
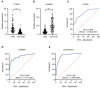
Results: From the study, we found that upregulated sphingosine-1-phosphate receptor 5 (S1PR5) and downregulated carnosine synthase 1 (CARNS1) had the most significant differences between the patient group and the control group. S1PR5 expression was correlated with diabetes, heart rate, triglycerides, total cholesterol, low-density lipoprotein cholesterol, apolipoprotein B, and fasting blood glucose (P < 0.05). CARNS1 level was correlated with uric acid (UA) (P < 0.05). Overexpressed S1PR5 and downregulated CARNS1 were independent risk factors for CHD. The area under the receiver operating characteristic curve (AUC) of S1PR5 was 0.838 for diagnosing CHD; the AUC of CARNS1 was 0.883 for non-CHD; and the AUC of S1PR5 plus CARNS1 was 0.921 for CHD.
Conclusions: Microarray analysis showed that upregulated S1PR5 and downregulated CARNS1 in sEVs have the potential to become non-invasive biomarkers for CHD screening.
Keywords: coronary heart disease; genomics; lipid metabolism; mRNA; sEVs.
Copyright © 2022 Xiong, Mao, Zhao, Zhang, Tan, Liu, Wang, Xu, Li and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

