Deciphering the Intercellular Communication Between Immune Cells and Altered Vascular Smooth Muscle Cell Phenotypes in Aortic Aneurysm From Single-Cell Transcriptome Data
- PMID: 35837612
- PMCID: PMC9273830
- DOI: 10.3389/fcvm.2022.936287
Deciphering the Intercellular Communication Between Immune Cells and Altered Vascular Smooth Muscle Cell Phenotypes in Aortic Aneurysm From Single-Cell Transcriptome Data
Abstract
Background: Vascular smooth muscle cell (VSMC) phenotype switching has been preliminarily found in aortic aneurysms. However, two major questions were raised: (1) What factors drive phenotypic switching of VSMCs in aortic aneurysms? (2) What role does VSMC phenotype transformation play in aortic aneurysms? We speculated that the interaction between infiltrated immune cells and VSMCs played a pivotal role in aortic aneurysm expansion.
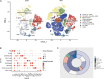
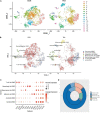
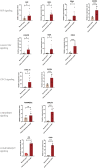
Materials and methods: We obtained single-cell transcriptome data GSE155468 that incorporate eight aortic aneurysm samples and three normal aorta samples. A standard single-cell analysis procedure was performed by Seurat (v3.1.2) for identifying the general cell components. Subsequently, VSMCs were extracted separately and re-clustered for identifying switched VSMC phenotypes. VSMC phenotype annotation was relied on the definitions of specific VSMC phenotypes in published articles. Vital VSMC phenotypes were validated by immunofluorescence. Next, identified immune cells and annotated vital VSMC phenotypes were extracted for analyzing the intercellular communication. R package CellChat (v1.1.3) was used for investigating the communication strength, signaling pathways, and communication patterns between various VSMC phenotypes and immune cells.
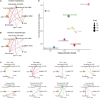
Result: A total of 42,611 cells were identified as CD4 + T cells, CD8 + T cells, VSMC, monocytes, macrophages, fibroblasts, endothelial cells, and B cells. VSMCs were further classified into contractile VSMCs, secreting VSMCs, macrophage-like VSMCs, mesenchymal-like VSMCs, adipocyte-like VSMCs, and T-cell-like VSMCs. Intercellular communication analysis was performed between immune cells (macrophages, B cells, CD4 + T cells, CD8 + T cells) and immune related VSMCs (macrophage-like VSMCs, mesenchymal-like VSMCs, T-cell-like VSMCs, contractile VSMCs). Among selected cell populations, 27 significant signaling pathways with 61 ligand-receptor pairs were identified. Macrophages and macrophage-like VSMCs both assume the roles of a signaling sender and receiver, showing the highest communication capability. T cells acted more as senders, while B cells acted as receivers in the communication network. T-cell-like VSMCs and contractile VSMCs were used as senders, while mesenchymal-like VSMCs played a poor role in the communication network. Signaling macrophage migration inhibitory factor (MIF), galectin, and C-X-C motif chemokine ligand (CXCL) showed high information flow of intercellular communication, while signaling complement and chemerin were completely turned on in aortic aneurysms. MIF and galectin promoted VSMC switch into macrophage-like phenotypes, CXCL, and galectin promoted VSMCs transform into T-cell-like phenotypes. MIF, galectin, CXCL, complement, and chemerin all mediated the migration and recruitment of immune cells into aortic aneurysms.
Conclusion: The sophisticated intercellular communication network existed between immune cells and immune-related VSMCs and changed as the aortic aneurysm progressed. Signaling MIF, galectin, CXCL, chemerin, and complement made a significant contribution to aortic aneurysm progression through activating immune cells and promoting immune cell migration, which could serve as the potential target for the treatment of aortic aneurysms.
Keywords: VSMC phenotype switching; aortic aneurysm; immune cells; intercellular communication; single-cell transcriptome.
Copyright © 2022 Cao, Lu, Gu, Xuan, Zhang, Hu and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bradbury AW, Davies AH, Dhesi JK, Hammond CJ, Hampshire M, Jellett K, et al. Recommendations on the use of open surgical and endovascular aneurysm repair for the management of unruptured abdominal aortic aneurysm from the guideline development committee appointed by the UK National Institute for Health and Care Excellence. Eur J Vasc Endovasc Surg. (2021) 61:877–80. 10.1016/j.ejvs.2021.01.047 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

