Detection and Quantification of ctDNA for Longitudinal Monitoring of Treatment in Non-Small Cell Lung Cancer Patients Using a Universal Mutant Detection Assay by Denaturing Capillary Electrophoresis
- PMID: 35837614
- PMCID: PMC9274771
- DOI: 10.3389/pore.2022.1610308
Detection and Quantification of ctDNA for Longitudinal Monitoring of Treatment in Non-Small Cell Lung Cancer Patients Using a Universal Mutant Detection Assay by Denaturing Capillary Electrophoresis
Abstract
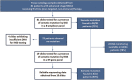
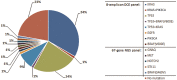
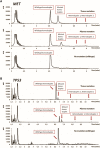
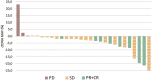
Background: Observation of anticancer therapy effect by monitoring of minimal residual disease (MRD) is becoming an important tool in management of non-small cell lung cancer (NSCLC). The approach is based on periodic detection and quantification of tumor-specific somatic DNA mutation in circulating tumor DNA (ctDNA) extracted from patient plasma. For such repetitive testing, complex liquid-biopsy techniques relying on ultra-deep NGS sequencing are impractical. There are other, cost-effective, methods for ctDNA analysis, typically based on quantitative PCR or digital PCR, which are applicable for detecting specific individual mutations in hotspots. While such methods are routinely used in NSCLC therapy prediction, however, extension to cover broader spectrum of mutations (e.g., in tumor suppressor genes) is required for universal longitudinal MRD monitoring. Methods: For a set of tissue samples from 81 NSCLC patients we have applied a denaturing capillary electrophoresis (DCE) for initial detection of somatic mutations within 8 predesigned PCR amplicons covering oncogenes and tumor suppressor genes. Mutation-negative samples were then subjected to a large panel NGS sequencing. For each patient mutation found in tissue was then traced over time in ctDNA by DCE. Results: In total we have detected a somatic mutation in tissue of 63 patients. For those we have then prospectively analyzed ctDNA from collected plasma samples over a period of up to 2 years. The dynamics of ctDNA during the initial chemotherapy therapy cycles as well as in the long-term follow-up matched the clinically observed response. Conclusion: Detection and quantification of tumor-specific mutations in ctDNA represents a viable complement to MRD monitoring during therapy of NSCLC patients. The presented approach relying on initial tissue mutation detection by DCE combined with NGS and a subsequent ctDNA mutation testing by DCE only represents a cost-effective approach for its routine implementation.
Keywords: KRAS mutations; NSCLC; TP53 mutations; capillary electrophoresis; ctDNA; liquid biopsy; minimal residual disease.
Copyright © 2022 Benesova, Ptackova, Halkova, Semyakina, Svaton, Fiala, Pesek and Minarik.
Conflict of interest statement
MM is employed by Elphogene company. Elphogene is currently providing oncoMonitor liquid biopsy/ctDNA test, which is in part utilizing the DCE mutation detection technology presented in this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Detection of Therapeutically Targetable Driver and Resistance Mutations in Lung Cancer Patients by Next-Generation Sequencing of Cell-Free Circulating Tumor DNA.Clin Cancer Res. 2016 Dec 1;22(23):5772-5782. doi: 10.1158/1078-0432.CCR-16-1231. Epub 2016 Sep 6. Clin Cancer Res. 2016. PMID: 27601595 Free PMC article.
-
High concordance of actionable genomic alterations identified between circulating tumor DNA-based and tissue-based next-generation sequencing testing in advanced non-small cell lung cancer: The Korean Lung Liquid Versus Invasive Biopsy Program.Cancer. 2021 Aug 15;127(16):3019-3028. doi: 10.1002/cncr.33571. Epub 2021 Apr 7. Cancer. 2021. PMID: 33826761
-
Heterogeneous mutation pattern in tumor tissue and circulating tumor DNA warrants parallel NGS panel testing.Mol Cancer. 2018 Aug 28;17(1):131. doi: 10.1186/s12943-018-0875-0. Mol Cancer. 2018. PMID: 30153823 Free PMC article.
-
Making the Rounds: Exploring the Role of Circulating Tumor DNA (ctDNA) in Non-Small Cell Lung Cancer.Int J Mol Sci. 2022 Aug 12;23(16):9006. doi: 10.3390/ijms23169006. Int J Mol Sci. 2022. PMID: 36012272 Free PMC article. Review.
-
Unlocking the future of cancer diagnosis - promises and challenges of ctDNA-based liquid biopsies in non-small cell lung cancer.Transl Res. 2024 Oct;272:41-53. doi: 10.1016/j.trsl.2024.05.014. Epub 2024 Jun 3. Transl Res. 2024. PMID: 38838851 Review.
Cited by
-
[Research progress on circulating tumor DNA as a biomarker for minimal residual disease in solid tumors].Zhongguo Dang Dai Er Ke Za Zhi. 2023 Oct 15;25(10):1072-1077. doi: 10.7499/j.issn.1008-8830.2304040. Zhongguo Dang Dai Er Ke Za Zhi. 2023. PMID: 37905766 Free PMC article. Review. Chinese.
References
-
- Theodoropoulos AS, Gkiozos I, Kontopyrgias G, Charpidou A, Kotteas E, Kyrgias G, et al. Modern Radiopharmaceuticals for Lung Cancer Imaging with Positron Emission Tomography/Computed Tomography Scan: A Systematic Review. SAGE Open Med (2020) 8:205031212096159. 10.1177/2050312120961594 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

