Evolocumab for prevention of microvascular dysfunction in patients undergoing percutaneous coronary intervention: the randomised, open-label EVOCATION trial
- PMID: 35837711
- PMCID: PMC10241273
- DOI: 10.4244/EIJ-D-22-00269
Evolocumab for prevention of microvascular dysfunction in patients undergoing percutaneous coronary intervention: the randomised, open-label EVOCATION trial
Abstract
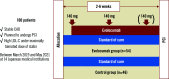
Statins have been shown to prevent microvascular dysfunction that may cause periprocedural myocardial infarction after percutaneous coronary intervention (PCI). Evolocumab has more potent lipid-lowering properties than statins. Aims: The aims of this study were to investigate whether evolocumab pretreatment on top of statin therapy could prevent periprocedural microvascular dysfunction. Methods: This study included 100 patients with stable coronary artery disease who were scheduled to undergo PCI and had high low-density lipoprotein cholesterol (LDL-C) under statin therapy. Patients were randomised to receive evolocumab 140 mg every 2 weeks for 2 to 6 weeks before PCI (evolocumab group: N=54) or not (control group: N=46). The primary endpoint was the index of microvascular resistance (IMR) after PCI. Troponin T was measured before and 24 hours after PCI. Results: Geometric mean LDL-C was 94.1 (95% confidence interval [CI]: 86.8-102.1) mg/dl and 89.4 (95% CI: 83.5-95.7) mg/dl at baseline, and 25.6 (95% CI: 21.9-30.0) mg/dl and 79.8 (95% CI: 73.9-86.3) mg/dl before PCI, in the evolocumab group and in the control group, respectively. PCI was performed 22.1±8.5 days after allocation. Geometric mean IMR was 20.6 (95% CI: 17.2-24.6) in the evolocumab group and 20.6 (95% CI: 17.0-25.0) in the control group (p=0.98). There was no significant difference in the geometric mean of post-PCI troponin T (0.054, 95% CI: 0.041-0.071 ng/ml vs 0.054, 95% CI: 0.038-0.077 ng/ml; p=0.99) and in the incidence of major periprocedural myocardial infarction between the 2 groups (44.4% vs 44.2%; p=1.00). Conclusions: Evolocumab pretreatment did not prevent periprocedural microvascular dysfunction in patients on modern medical management with statins.
Conflict of interest statement
K. Tsujita received remuneration for lectures from Amgen K.K. All other authors have reported that they have no conflicts of interest to declare relevant to the contents of this paper.
Figures



Similar articles
-
Design and rationale of the EVOCATION trial: A prospective, randomized, exploratory study comparing the effect of evolocumab on coronary microvascular function after percutaneous coronary intervention in patients with stable coronary artery disease.J Cardiol. 2022 Jan;79(1):105-109. doi: 10.1016/j.jjcc.2021.08.024. Epub 2021 Sep 10. J Cardiol. 2022. PMID: 34518072 Clinical Trial.
-
Early Initiation of Evolocumab Treatment in Chinese Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention.Clin Ther. 2022 Jun;44(6):901-912. doi: 10.1016/j.clinthera.2022.04.010. Epub 2022 May 14. Clin Ther. 2022. PMID: 35581018
-
Effect of Evolocumab in Patients With Prior Percutaneous Coronary Intervention.Circ Cardiovasc Interv. 2022 Mar;15(3):e011382. doi: 10.1161/CIRCINTERVENTIONS.121.011382. Epub 2022 Feb 25. Circ Cardiovasc Interv. 2022. PMID: 35209731 Clinical Trial.
-
Pharmacological rationale for the very early treatment of acute coronary syndrome with monoclonal antibodies anti-PCSK9.Pharmacol Res. 2022 Oct;184:106439. doi: 10.1016/j.phrs.2022.106439. Epub 2022 Sep 12. Pharmacol Res. 2022. PMID: 36100012 Review.
-
High dose statin loading prior to percutaneous coronary intervention decreases cardiovascular events: a meta-analysis of randomized controlled trials.Catheter Cardiovasc Interv. 2015 Jan 1;85(1):53-60. doi: 10.1002/ccd.25302. Epub 2013 Dec 21. Catheter Cardiovasc Interv. 2015. PMID: 24272891 Review.
Cited by
-
The imPAct of Trimetazidine on MicrOcirculation after Stenting for stable coronary artery disease (PATMOS study).Front Cardiovasc Med. 2023 Jun 30;10:1112198. doi: 10.3389/fcvm.2023.1112198. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37456821 Free PMC article.
-
Alirocumab and chest pain after acute coronary syndrome: An analysis of ODYSSEY OUTCOMES.Am J Prev Cardiol. 2024 Nov 26;20:100900. doi: 10.1016/j.ajpc.2024.100900. eCollection 2024 Dec. Am J Prev Cardiol. 2024. PMID: 39687787 Free PMC article.
-
Rationale for Early Administration of PCSK9 Inhibitors in Acute Coronary Syndrome.Rev Cardiovasc Med. 2024 Oct 22;25(10):374. doi: 10.31083/j.rcm2510374. eCollection 2024 Oct. Rev Cardiovasc Med. 2024. PMID: 39484117 Free PMC article. Review.
-
In search of the pleiotropic effects of PCSK9 inhibitors.EuroIntervention. 2022 Oct 7;18(8):e609-e610. doi: 10.4244/EIJ-E-22-00032. EuroIntervention. 2022. PMID: 36205734 Free PMC article. No abstract available.
-
Present and Future of Dyslipidaemia Treatment-A Review.J Clin Med. 2023 Sep 8;12(18):5839. doi: 10.3390/jcm12185839. J Clin Med. 2023. PMID: 37762780 Free PMC article. Review.
References
-
- Navarese EP, Lansky AJ, Kereiakes DJ, Kubica J, Gurbel PA, Gorog DA, Valgimigli M, Curzen N, Kandzari DE, Bonaca MP, Brouwer M, Umińska J, Jaguszewski MJ, Raggi P, Waksman R, Leon MB, Wijns W, Andreotti F. Cardiac mortality in patients randomised to elective coronary revascularisation plus medical therapy or medical therapy alone: a systematic review and meta-analysis. Eur Heart J. 2021;42:4638–51. - PMC - PubMed
-
- Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink JH, Rioufol G, Toth GG, Piroth Z, Witt N, Fröbert O, Kala P, Linke A, Jagic N, Mates M, Mavromatis K, Samady H, Irimpen A, Oldroyd K, Campo G, Rothenbühler M, Jüni P, De Bruyne FAME 2 Investigators. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N Engl J Med. 2018;379:250–9. - PubMed
-
- Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, Chaitman BR, Senior R, López-Sendón J, Alexander KP, Lopes RD, Shaw LJ, Berger JS, Newman JD, Sidhu MS, Goodman SG, Ruzyllo W, Gosselin G, Maggioni AP, White HD, Bhargava B, Min JK, Mancini G, Berman DS, Picard MH, Kwong RY, Ali ZA, Mark DB, Spertus JA, Krishnan MN, Elghamaz A, Moorthy N, Hueb WA, Demkow M, Mavromatis K, Bockeria O, Peteiro J, Miller TD, Szwed H, Doerr R, Keltai M, Selvanayagam JB, Steg PG, Held C, Kohsaka S, Mavromichalis S, Kirby R, Jeffries NO, Harrell FE, Rockhold FW, Broderick S, Ferguson TB, Williams DO, Harrington RA, Stone GW, Rosenberg Y ISCHEMIA Research Group. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395–407. - PMC - PubMed
-
- Gregson J, Stone GW, Ben-Yehuda O, Redfors B, Kandzari DE, Morice MC, Leon MB, Kosmidou I, Lembo NJ, Brown WM, Karmpaliotis D, Banning AP, Pomar J, Sabaté M, Simonton CA, Dressler O, Kappetein AP, Sabik JF, Serruys PW, Pocock SJ. Implications of Alternative Definitions of Peri-Procedural Myocardial Infarction After Coronary Revascularization. J Am Coll Cardiol. 2020;76:1609–21. - PubMed
-
- Hara H, Serruys PW, Takahashi K, Kawashima H, Ono M, Gao C, Wang R, Mohr FW, Holmes DR, Davierwala PM, Head SJ, Thuijs DJFM, Milojevic M, Kappetein AP, Garg S, Onuma Y, Mack MJ SYNTAX Extended Survival Investigators. Impact of Peri-Procedural Myocardial Infarction on Outcomes After Revascularization. J Am Coll Cardiol. 2020;76:1622–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

