Anaemia and pathologic complete response rate according to carboplatin dose in HER2+ breast cancer treated with neoadjuvant TCHP
- PMID: 35837812
- PMCID: PMC9883435
- DOI: 10.1002/cam4.5022
Anaemia and pathologic complete response rate according to carboplatin dose in HER2+ breast cancer treated with neoadjuvant TCHP
Abstract
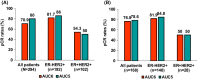
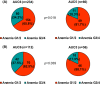
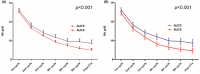
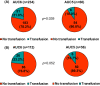
Grade 3/4 anaemia, which is mainly induced by carboplatin, frequently occurs in patients treated with neoadjuvant docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP). However, dose reduction of carboplatin may raise concerns about the oncological outcome. This study investigated the pathologic complete response (pCR) rate, occurrence of grade 3/4 anaemia, and transfusion rate according to carboplatin dose in patients treated with neoadjuvant TCHP. We retrospectively analysed 294 patients treated with neoadjuvant TCHP between April 2015 and December 2020. Case matching was performed using propensity score matching. Among patients treated with neoadjuvant TCHP, carboplatin area under the plasma concentration-time curve 6 (AUC6) was used in 234 patients (79.6%) and upfront carboplatin AUC5 was used in 60 patients (20.4%). No significant difference in pCR rate was found between the two groups (AUC6: 70.9%, AUC5: 80.0%). In both oestrogen receptor-positive (ER+) and ER- patients, no significant differences were observed between the AUC6 and AUC5 groups (ER+: 54.3% vs. 50.0%, ER-: 81.7% vs. 86.0%). The case-matched cohort showed consistent findings. The AUC5 group had lower frequencies of grade 3/4 anaemia (18.3% vs. 34.2%) and transfusion events (10.0% vs. 21.8%) than the AUC6 group. Compared with AUC5, carboplatin at AUC6 would associate with a 2.7-fold increased risk of grade 3 or 4 chemotherapy-induced anaemia. Carboplatin AUC5 has comparable cytotoxic effects to carboplatin AUC6 in patients with HER2+ breast cancer treated with six cycles of neoadjuvant TCHP, with fewer complications associated with clinically meaningful anaemia. AUC5 may be the optimal carboplatin dose to reduce TCHP-induced anaemia in patients with HER2+ breast cancer treated with TCHP.
Keywords: anaemia; breast cancer; carboplatin; neoadjuvant chemotherapy; pathologic complete response.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None of the co‐authors have any potential competing interests.
Figures
Similar articles
-
Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial.Lancet Oncol. 2018 Jan;19(1):115-126. doi: 10.1016/S1470-2045(17)30716-7. Epub 2017 Nov 23. Lancet Oncol. 2018. PMID: 29175149 Clinical Trial.
-
Carboplatin dose capping affects pCR rate in HER2-positive breast cancer patients treated with neoadjuvant Docetaxel, Carboplatin, Trastuzumab, Pertuzumab (TCHP).Breast Cancer Res Treat. 2020 Nov;184(2):481-489. doi: 10.1007/s10549-020-05868-z. Epub 2020 Aug 29. Breast Cancer Res Treat. 2020. PMID: 32860550 Free PMC article.
-
Impact of Dose Intensity on Pathologic Complete Response Rate in HER2-Positive Breast Cancer Patients Receiving Neoadjuvant Docetaxel, Carboplatin, Trastuzumab and Pertuzumab (TCHP).Target Oncol. 2022 Mar;17(2):167-175. doi: 10.1007/s11523-022-00874-1. Epub 2022 Mar 24. Target Oncol. 2022. PMID: 35325355
-
Neoadjuvant weekly paclitaxel and carboplatin with trastuzumab and pertuzumab in HER2-positive breast cancer: a Brown University Oncology Research Group (BrUOG) study.Breast Cancer Res Treat. 2021 Aug;189(1):93-101. doi: 10.1007/s10549-021-06266-9. Epub 2021 Jun 4. Breast Cancer Res Treat. 2021. PMID: 34086171 Clinical Trial.
-
A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients.Breast Cancer Res Treat. 2019 Jan;173(2):255-266. doi: 10.1007/s10549-018-4981-x. Epub 2018 Oct 15. Breast Cancer Res Treat. 2019. PMID: 30324273 Review.
Cited by
-
Predictive Markers of Treatment Response to Neoadjuvant Systemic Therapy with Dual HER2-Blockade.Cancers (Basel). 2024 Feb 19;16(4):842. doi: 10.3390/cancers16040842. Cancers (Basel). 2024. PMID: 38398233 Free PMC article.
-
De-escalation of neoadjuvant taxane and carboplatin therapy in HER2-positive breast cancer with dual HER2 blockade: a multicenter real-world experience in China.World J Surg Oncol. 2024 Aug 21;22(1):214. doi: 10.1186/s12957-024-03468-5. World J Surg Oncol. 2024. PMID: 39164763 Free PMC article.
References
-
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997‐4013. doi:10.1200/jco.2013.50.9984 - DOI - PubMed
-
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2‐positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2‐negative cohort. Lancet. 2010;375:377‐384. doi:10.1016/s0140-6736(09)61964-4 - DOI - PubMed
-
- Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2‐overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351‐3357. doi:10.1200/jco.2010.31.4930 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

