CD11b suppresses TLR activation of nonclassical monocytes to reduce primary graft dysfunction after lung transplantation
- PMID: 35838047
- PMCID: PMC9282933
- DOI: 10.1172/JCI157262
CD11b suppresses TLR activation of nonclassical monocytes to reduce primary graft dysfunction after lung transplantation
Abstract
Primary graft dysfunction (PGD) is the leading cause of postoperative mortality in lung transplant recipients and the most important risk factor for development of chronic lung allograft dysfunction. The mechanistic basis for the variability in the incidence and severity of PGD between lung transplant recipients is not known. Using a murine orthotopic vascularized lung transplant model, we found that redundant activation of Toll-like receptors 2 and 4 (TLR2 and -4) on nonclassical monocytes activates MyD88, inducing the release of the neutrophil attractant chemokine CXCL2. Deletion of Itgam (encodes CD11b) in nonclassical monocytes enhanced their production of CXCL2 and worsened PGD, while a CD11b agonist, leukadherin-1, administered only to the donor lung prior to lung transplantation, abrogated CXCL2 production and PGD. The damage-associated molecular pattern molecule HMGB1 was increased in peripheral blood samples from patients undergoing lung transplantation after reperfusion and induced CXCL2 production in nonclassical monocytes via TLR4/MyD88. An inhibitor of HMGB1 administered to the donor and recipient prior to lung transplantation attenuated PGD. Our findings suggest that CD11b acts as a molecular brake to prevent neutrophil recruitment by nonclassical monocytes following lung transplantation, revealing an attractive therapeutic target in the donor lung to prevent PGD in lung transplant recipients.
Keywords: Immunology; Integrins; Monocytes; Transplantation.
Conflict of interest statement
Figures

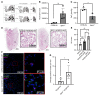


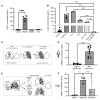
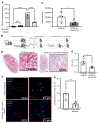
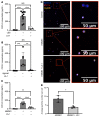

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

